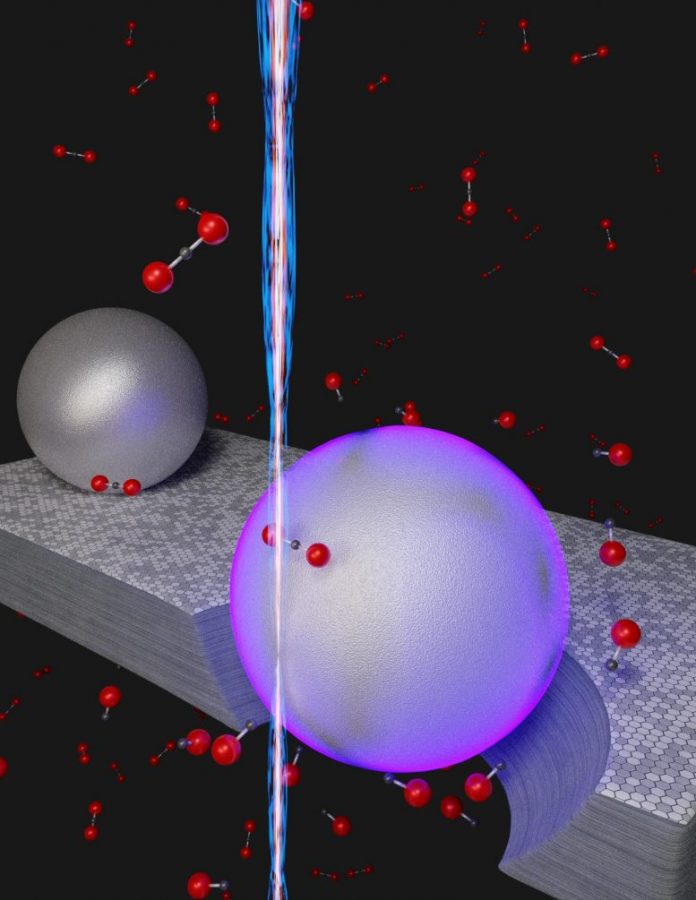
Illustration of an unique room-temperature procedure to eliminate co2 (CO2) by transforming the particle into carbon monoxide gas (CO). Instead of utilizing heat, the nanoscale technique counts on the energy from surface area plasmons (violet shade) that are thrilled when a beam of electrons (vertical beam) strikes aluminum nanoparticles resting on graphite, a crystalline type of carbon. In the existence of the graphite, assisted by the energy originated from the plasmons, co2 particles (black dot bonded to 2 red dots) are transformed to carbon monoxide gas (black dot bonded to one red dot. The hole under the violet sphere represents the graphite engraved away throughout the chain reaction CO2 + C = 2CO. Credit: NIST
Room temperature level conversion of CO2 to CO
Researchers at the National Institute of Standards and Technology (NIST) and their coworkers have actually shown a room-temperature technique that might considerably decrease co2 levels in fossil-fuel power plant exhaust, among the primary sources of carbon emissions in the environment.
Although the scientists showed this technique in a small, extremely regulated environment with measurements of simply nanometers (billionths of a meter), they have actually currently developed principles for scaling up the technique and making it useful for real-world applications.
In addition to using a possible brand-new method of reducing the results of environment modification, the chemical procedure used by the researchers likewise might decrease expenses and energy requirements for producing liquid hydrocarbons and other chemicals utilized by market. That’s due to the fact that the technique’s by-products consist of the foundation for manufacturing methane, ethanol and other carbon-based substances utilized in commercial processing.
The group tapped an unique energy source from the nanoworld to activate an ordinary chain reaction that removes co2. In this response, strong carbon acquires among the oxygen atoms in co2 gas, decreasing it to carbon monoxide gas. The conversion usually needs considerable quantities of energy in the type of high heat — a temperature level of a minimum of 700 degrees Celsius, hot enough to melt aluminum at regular air pressure.
Instead of heat, the group counted on the energy collected from taking a trip waves of electrons, called localized surface area plasmons (LSPs), which browse on private aluminum nanoparticles. The group set off the LSP oscillations by amazing the nanoparticles with an electron beam that had an adjustable size. A narrow beam, about a nanometer in size, bombarded private aluminum nanoparticles while a beam about a thousand times broader produced LSPs amongst a big set of the nanoparticles.
In the group’s experiment, the aluminum nanoparticles were transferred on a layer of graphite, a kind of carbon. This enabled the nanoparticles to move the LSP energy to the graphite. In the existence of co2 gas, which the group injected into the system, the graphite served the function of plucking private oxygen atoms from co2, decreasing it to carbon monoxide gas. The aluminum nanoparticles were kept at space temperature level. In by doing this, the group achieved a significant task: eliminating the co2 without the requirement for a source of high heat.
Previous techniques of getting rid of co2 have actually had restricted success due to the fact that the strategies have actually needed heat or pressure, used expensive rare-earth elements, or had bad performance. In contrast, the LSP technique not just conserves energy however utilizes aluminum, an inexpensive and plentiful metal.
Although the LSP response creates a toxic gas — carbon monoxide gas — the gas easily integrates with hydrogen to produce necessary hydrocarbon substances, such as methane and ethanol, that are frequently utilized in market, stated NIST scientist Renu Sharma.
She and her coworkers, consisting of researchers from the University of Maryland in College Park and DENSsolutions, in Delft, the Netherlands, reported their findings in Nature Materials.
“We showed for the first time that this carbon dioxide reaction, which otherwise will only happen at 700 degrees C or higher, can be triggered using LSPs at room temperature,” stated scientist Canhui Wang of NIST and the University of Maryland.
The scientists selected an electron beam to thrill the LSPs due to the fact that the beam can likewise be utilized to image structures in the system as little as a couple of billionths of a meter. This made it possible for the group to approximate just how much co2 had actually been eliminated. They studied the system utilizing a transmission electron microscopic lense (TEM).
Because both the concentration of co2 and the response volume of the experiment were so little, the group needed to take unique actions to straight determine the quantity of carbon monoxide gas produced. They did so by coupling a specifically customized gas cell holder from the TEM to a gas chromatograph mass spectrometer, enabling the group to determine parts-per-millions concentrations of co2.
Sharma and her coworkers likewise utilized the images produced by the electron beam to determine the quantity of graphite that was engraved away throughout the experiment, a proxy for just how much co2 had actually been eliminated. They discovered that the ratio of carbon monoxide gas to co2 determined at the outlet of the gas cell holder increased linearly with the quantity of carbon eliminated by etching.
Imaging with the electron beam likewise verified that the majority of the carbon etching — a proxy for co2 decrease — took place near the aluminum nanoparticles. Additional research studies exposed that when the aluminum nanoparticles were missing from the experiment, just about one-seventh as much carbon was engraved.
Limited by the size of the electron beam, the group’s speculative system was little, just about 15 to 20 nanometers throughout (the size of a little infection).
To scale up the system so that it might eliminate co2 from the exhaust of a business power plant, a beam might be a much better option than an electron beam to thrill the LSPs, Wang stated. Sharma proposes that a transparent enclosure consisting of loosely jam-packed carbon and aluminum nanoparticles might be put over the smokestack of a power plant. An selection of beams impinging upon the grid would trigger the LSPs. When the exhaust travels through the gadget, the light-activated LSPs in the nanoparticles would offer the energy to eliminate co2.
The aluminum nanoparticles, which are commercially readily available, need to be equally dispersed to take full advantage of contact with the carbon source and the inbound co2, the group kept in mind.
The brand-new work likewise recommends that LSPs use a method for a variety of other chain reactions that now need a big infusion of energy to continue at common temperature levels and pressures utilizing plasmonic nanoparticles.
“Carbon dioxide reduction is a big deal, but it would be an even bigger deal, saving enormous amounts of energy, if we can start to do many chemical reactions at room temperature that now require heating,” Sharma stated.
Reference: “Endothermic Reaction at Room Temperature enabled by Deep-Ultraviolet Plasmons” by Canhui Wang, Wei-Chang D. Yang, David Raciti, Alina Bruma, Ronald Marx, Amit Agrawal, and Renu Sharma, 2 November 2020, Nature Materials.
DOI: 10.1038/s41563-020-00851-x