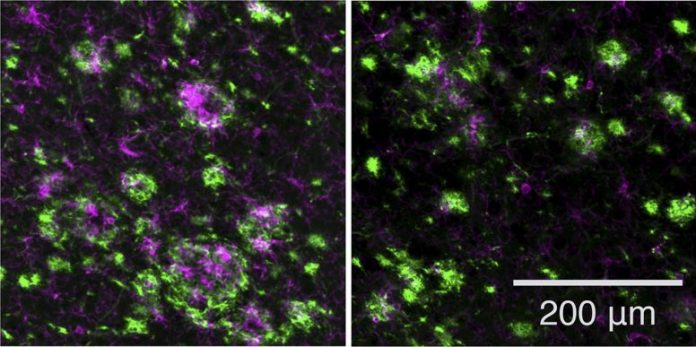
Compared with a control (left), treatment with the unique GSM (right) decreases the variety of amyloid plaques (green) and proinflammatory cells called microglia (magenta) in the brains of mice bring anomalies connected to early-onset Alzheimer’s illness. Credit: ©2021 Rynearson et al. Originally released in Journal of Experimental Medicine. DOI 10.1084/jem.20202560
Researchers at the University of California San Diego School of Medicine and Massachusetts General Hospital have actually recognized a brand-new drug that might avoid Alzheimer’s illness by regulating, instead of hindering, an essential enzyme associated with forming amyloid plaques in the brain. The research study, which will be released today (March 2, 2021) in the Journal of Experimental Medicine (JEM), shows that the drug is safe and reliable in rodents and monkeys, leading the way for future scientific trials in human beings.
A crucial pathological trademark of Alzheimer’s illness includes the development of amyloid plaques made up of little protein pieces referred to as amyloid-β (Aβ) peptides. These peptides are created by enzymes called β-secretase and γ-secretase, which sequentially cleave a protein called amyloid precursor protein on the surface area of nerve cells to launch Aβ pieces of differing lengths. Some of these pieces, such as Aβ42, are especially vulnerable to forming amyloid plaques, and their production rises in clients with anomalies inclining them to early-onset Alzheimer’s illness.
Several efforts have actually been made to deal with or avoid Alzheimer’s illness utilizing drugs that prevent either β-secretase or γ-secretase. But a lot of these drugs have actually shown to be risky in human beings, likely since β-secretase and γ-secretase are needed to cleave extra proteins in the brain and other organs. A much better method might include drugs referred to as γ-secretase modulators (GSMs), which, rather of hindering the γ-secretase enzyme, somewhat modify its activity so that it produces less Aβ peptides that are vulnerable to form plaques while continuing to cleave its other protein targets.
“GSMs therefore offer the ability to mitigate mechanism-based toxicities associated with γ-secretase inhibitors,” states Steven L. Wagner, PhD, a teacher in the Department of Neurosciences at UC San Diego School of Medicine.
In the brand-new research study, Wagner and coworkers established an unique GSM and evaluated it on mice, rats, and macaques. Repeated low dosages of the drug totally removed Aβ42 production in mice and rats without triggering any harmful adverse effects. The drug was likewise safe and reliable in macaques, lowering Aβ42 levels by approximately 70%.
The scientists then evaluated the drug in a mouse design of early-onset Alzheimer’s illness, dealing with the animals either prior to or quickly after they started to form amyloid plaques. In both cases, the unique GSM reduced plaque development and minimized plaque-associated swelling, which is believed to add to the advancement of illness.
This recommends that the drug might be utilized prophylactically to avoid Alzheimer’s illness, either in clients with hereditary anomalies that increase vulnerability to the illness or in cases where amyloid plaques have actually been spotted by brain scans.
“In this study, we have pharmacologically characterized a potent GSM that, based on its preclinical attributes, appears to equal or exceed the potency of any previously tested GSMs,” includes Dr. Rudolph E. Tanzi, Professor of Neurology at Harvard and Massachusetts General Hospital. “Future clinical trials will determine whether this promising GSM is safe in humans and could be used to effectively treat or prevent Alzheimer’s disease.”
Reference: “Preclinical Validation of a Potent γ-Secretase Modulator for Alzheimer’s Disease Prevention” by Kevin D. Rynearson, Moorthi Ponnusamy, Olga Prikhodko, Yuhuan Xie, Can Zhang, Phuong Nguyen, Brenda Hug, Mariko Sawa, Ann Becker, Brian Spencer, Jazmin Florio, Michael Mante, Bahar Salehi, Carlos Arias, Douglas Galasko, Brian P. Head, Graham Johnson, Jiunn H. Lin, Steven K. Duddy, Robert A. Rissman, William C. Mobley, Gopal Thinakaran, Rudolph E. Tanzi and Steven L. Wagner, 2 March 2021, Journal of Experimental Medicine.
DOI: 10.1084/jem.20202560