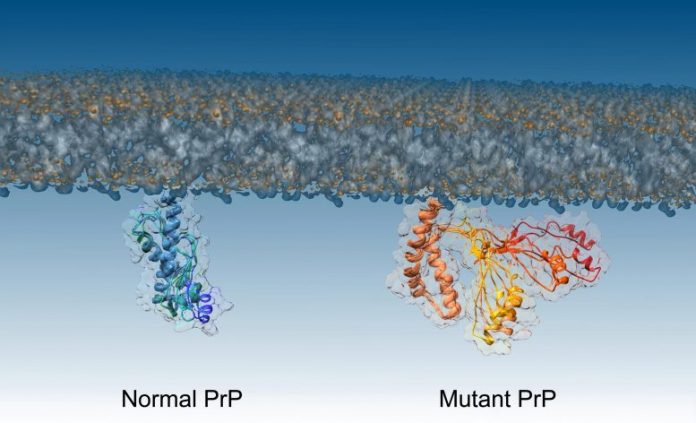
The structure of regular and mutant PrP proteins. Credit: Imperial College London
For the very first time, scientists have actually identified what triggers regular proteins to transform to an infected type, triggering conditions like CJD and Kuru.
The research study group, from Imperial College London and the University of Zurich, likewise checked a method to obstruct the procedure, which might result in brand-new drugs for fighting these illness.
The research study worried prion illness – a group of brain illness brought on by proteins called prions that breakdown and ‘misfold’, becoming a kind that can collect and eliminate brain cells. These illness can take years to manifest, however are then aggressive and deadly.
“Discovering the mechanism by which prions become pathogenic is a crucial step in one day tackling these diseases, as it allows us to search for new drugs.” — Professor Alfonso De Simone
They consist of Kuru, mad cow illness and its human equivalent Creutzfeldt-Jakob illness (CJD), and a heritable condition called deadly familial sleeping disorders.
While the regular, healthy variation of prions and the pathogenic (disease-causing) variation have actually been identified, the intermediate action, when one changes to the other, was formerly unidentified.
Now, in a paper released on March 15, 2021, in Proceedings of the National Academy of Sciences, the research study group have actually separated this intermediate action, identifying the system that turns regular prions into their pathogenic type. The research study was supported by Alzheimer’s Research UK.
Aggressive and ravaging
Lead scientist Professor Alfonso De Simone, from the Department of Life Sciences at Imperial, stated: “Prion illness are aggressive and disastrous, and presently there is no remedy.
“Discovering the mechanism by which prions become pathogenic is a crucial step in one day tackling these diseases, as it allows us to search for new drugs. Now we know what we’re targeting, we know what features drugs need to have to stop prions becoming pathogenic.”
To examine the misfolding of prions, the group dealt with a mutant type of the prion protein that is discovered in individuals with acquired prion illness. The mutant type is more aggressive, triggering prions to shift much faster to their pathogenic type. This permits the scientists to see what takes place more quickly.
However, prions are challenging to separate and cleanse from other proteins in adequate amounts to study in information. Lead author of the paper Dr. Máximo Sanz-Hernández started examining the issue as an undergrad at Imperial, continuing up until effective in his PostDoc with Professor De Simone.
The group then utilized a method called nuclear magnetic resonance spectroscopy integrated with computational analysis to identify the structure of the intermediate action, determining the molecular system at work when the prion misfolds.
With this info, they likewise dealt with the group at the University of Zurich who had the ability to produce antibodies that might target the system. In a proof-of-concept research study in the test tube, they were effectively able to obstruct prions transitioning from the regular to the pathogenic type.
While in their existing type, these antibodies would be too big to enter the brain, the research study reveals it is possible to interfere with the system, enabling scientists to move on with developing brand-new drugs.
Dr. Sanz-Hernández stated: “The intermediate stage of prion pathogenesis is so transient it’s like a ghost – almost impossible to image. But now we have a picture of what we’re dealing with, we can design more specific interventions that can one day potentially control these devastating diseases.”
Searching for drug substances
Dr. Rosa Sancho, Head of Research at Alzheimer’s Research UK, stated: “This is early-stage research study taking a look at the brief protein pieces, which can be extremely unsteady, brief lived, and infamously challenging to study.
“As the UK’s leading dementia research charity, we are pleased to fund this sophisticated work using biophysical and computational approaches to better understand the role fragments like this play in the development of disease. To identify new ways to reduce or combat these protein fragments in human disease we need to see sustained investment in dementia research.”
The scientists hope the info will permit drug scientists and pharmaceutical business to scan their libraries of drug substances for formulas that may be able to obstruct the system.
Any drug substances would require comprehensive laboratory screening initially to ensure they would work, little enough to enter the brain, and safe, however the group hope that now the target is understood, the search can be sped up.
Reference: “Mechanism of misfolding of the human prion protein revealed by a pathological mutation” by Máximo Sanz-Hernández, Joseph D. Barritt, Jens Sobe, Simone Hornemann, Adriano Aguzzi and Alfonso De Simone, 15 March 2021, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2019631118