A chemical-looping variation of the reverse water-gas shift response (RWGS-CL) can assist transform CO2 to CO at much lower temperature levels without unwanted by-products, allowing an easy gas separation. Credit: Yasushi Sekine from Waseda University
Scientists set a record for the greatest conversion rate of co2 at low temperature levels with copper-modified indium oxide, symbolizing sustainable e-fuel.
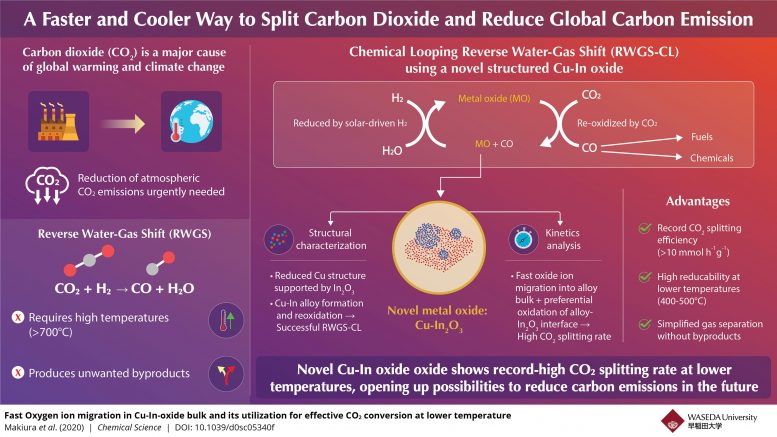
With ever-worsening environment modification, there is a growing requirement for innovations that can record and consume the climatic CO2 (co2) and minimize our carbon footprint. Within the world of renewable resource, CO2-based e-fuels have actually become an appealing innovation that tries to transform climatic CO2 into tidy fuels. The procedure includes production of artificial gas or syngas (a mix of hydrogen and carbon monoxide gas (CO)). With the assistance of the reverse water-gas shift (RWGS) response, CO2 is broken down into the CO needed for syngas. While appealing in its conversion performance, the RWGS response needs extremely heats (>700°C) to continue, while likewise creating undesirable by-products.
To take on these issues, researchers established a customized chemical-looping variation of the RWGS response that transforms CO2 to CO in a two-step technique. First, a metal oxide, utilized as an oxygen storage product, is minimized by hydrogen. Subsequently, it is re-oxidized by CO2, yielding CO. This technique is devoid of unwanted by-products, makes gas separation easier, and can be made possible at lower temperature levels depending upon the oxide picked. Consequently, researchers have actually been trying to find oxide products that display high oxidation-reduction rates without needing heats.
In a current research study released in Chemical Science, researchers from Waseda University and ENEOS Corporation in Japan have actually exposed that an unique indium oxide customized with copper (Cu-In2O3) shows a record-breaking CO2 conversion rate of 10 mmolh-1g-1 at fairly modest temperature levels (400–500°C), making it a frontrunner amongst oxygen storage products needed for low-temperature CO2 conversion. To much better comprehend this habits, the group examined the structural homes of Cu-In oxide together with the kinetics associated with the chemical-looping RWGS response.
A record-high CO2 conversion rates at fairly low temperature levels in a customized chemical-looping variation of RWGS utilizing an unique copper-indium oxide. Credit: Waseda University
The researchers performed X-ray-based analyses and discovered that the sample at first consisted of a moms and dad product, Cu2In2O5, which was very first minimized by hydrogen to form a Cu-In alloy and indium oxide (In2O3) and after that oxidized by CO2 to yield Cu-In2O3 and CO. X-ray information even more exposed that it went through oxidation and decrease throughout the response, offering the crucial hint to researchers. “The X-ray measurements made it clear that the chemically looped RWGS reaction is based on the reduction and oxidation of Indium which leads to the formation and oxidation of the Cu-In alloy,” discusses Professor Yasushi Sekine of Waseda University, who led the research study.
The kinetics examinations supplied even more insights into the response. The decrease action exposed that Cu was accountable for the decrease of indium oxide at low temperature levels, while the oxidation action revealed that the Cu-In alloy surface area protected an extremely minimized state while its bulk got oxidized. This enabled the oxidation to take place two times as rapidly as that of other oxides. The group associated this strange oxidation habits to a quick migration of adversely charged oxygen ions from the Cu-In alloy surface area to its bulk, which helped in the preferential bulk oxidation.
The outcomes have, rather expectedly, thrilled researchers about the future potential customers of copper-indium oxides. “Given the current situation with carbon emission and global warming, a high-performance carbon dioxide conversion process is greatly desired. Although the chemically looped RWGS reaction works well with many oxide materials, our novel Cu-In-oxide here shows a remarkably higher performance than any of them. We hope that this will contribute significantly to reducing our carbon footprint and driving humankind towards a more sustainable future,” concludes Sekine.
Reference: “Fast oxygen ion migration in Cu–In–oxide bulk and its usage for reliable CO2 conversion at lower temperature level” by Jun-Ichiro Makiura, Takuma Higo, Yutaro Kurosawa, Kota Murakami, Shuhei Ogo, Hideaki Tsuneki, Yasushi Hashimoto, Yasushi Satob and Yasushi Sekine, 23 December 2020, Chemical Science.
DOI: 10.1039/d0sc05340f





