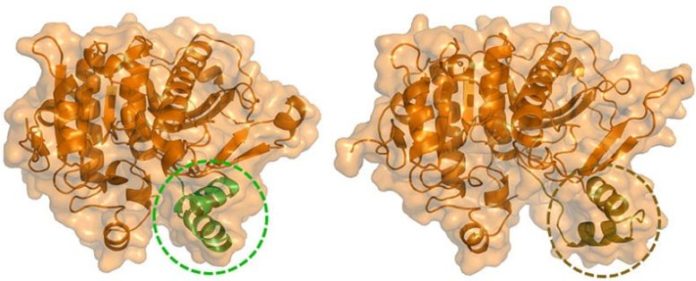
Lp- PLA 2 in its “closed” conformation (left) and “open” conformation (right) as soon as bound to the phospholipid monolayer. Credit: UC San Diego Health Sciences
New simulations expose the system of action and substrate uniqueness of lipoprotein-associated phospholipase A2, a biomarker for heart disease.
Membrane- associated proteins play a crucial function in a range of cellular procedures, yet little is learnt about the membrane-association system. Lipoprotein- associated phospholipase A 2 (Lp- PLA 2) is one such protein with an essential function in cardiovascular health, however its system of action on the phospholipid membrane was unidentified. To address this, scientists at University of California San Diego School of Medicine utilized advanced speculative and computational tools to reveal precisely how the enzyme engages with the membrane and extracts its particular substrates.
The findings were released on January 3, 2022, in the online concern of Proceedings of the National Academy of Sciences
Lp- PLA 2 deals with lipoproteins in the blood stream, consisting of typical types like low- and high-density lipoprotein (LDL and HDL). These lipoprotein particles are comprised of a round layer of phospholipids surrounding a drop of fat and cholesterol esters. Over time, the phospholipids in this external layer ended up being oxidized, drawing in totally free radicals and additional oxidation, which adds to plaque accumulation and heart disease.
Lp- PLA 2 extracts these oxidized phospholipids from the lipoprotein membrane and launches their fats to be additional metabolized. Understanding precisely how this procedure works develops brand-new chances for rehabs versus heart disease.
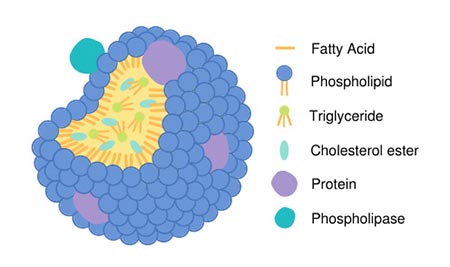
Schematic of a common lipoprotein with a phospholipase bound to the external phospholipid monolayer. Credit: UC San Diego Health Sciences
“I am very pleased that we were able to go into much greater depth on how this enzyme works than ever before,” stated Edward A. Dennis, PhD, senior author of the research study and Distinguished Professor of Pharmacology, Chemistry and Biochemistry at UC San Diego School ofMedicine “Using the latest advances in lipidomics and computational molecular dynamics simulations, we got a picture which is worth a thousand words. We now have movies that show how this enzyme works at the atomic level, and that should help us figure out ways to activate or inactivate the enzyme as necessary for health.”
This advanced technique exposed a particular peptide area including 2 alpha helices gotten in touch with a loop that serves as a gate to the enzyme’s active website. Typically, this gate remains in a “closed” position, however when Lp- PLA 2 binds to the phospholipid membrane, it goes through an allosteric conformational modification that opens eviction and increases the volume of the active website.
Dennis’ group, led by very first author Varnavas D. Mouchlis, PhD, likewise revealed which oxidized phospholipid substrates Lp- PLA 2 has the best affinity for. They even more determined a binding pocket unique from understood drug inhibitor binding pockets, which might act as a brand-new target for future healing drugs.
This research study is the current in a long profession from the Dennis laboratory to establish a unifying theory on the function of phospholipases. The group had actually formerly presented this idea of membrane-facilitated allosteric policy of PLA 2 enzymes, however had up until this point just studied enzymes that operate on phospholipid bilayers (as seen on cells and intracellular organelles). This research study verified that a comparable system might be utilized to help with phospholipase action on phospholipid monolayers, such as those on lipoproteins.
” PLA 2 enzymes have all sorts of crucial functions in swelling, food digestion, brain health, and more, so it’s incredible to see this wide array of enzymes all reveal a comparable action technique,” statedDennis “We’ve been studying this superfamily of enzymes for almost 50 years, so to finally have this complete picture of how they work is really satisfying, and the whole field advances.”
Reference: 3 January 2022, Proceedings of the National Academy of Sciences
DOI: 10.1073/ pnas.2102953119
Co- authors consist of: Daiki Hayashi, Alexis M. Vazquez, Jian Cao and J. Andrew McCammon, all at UC San Diego.