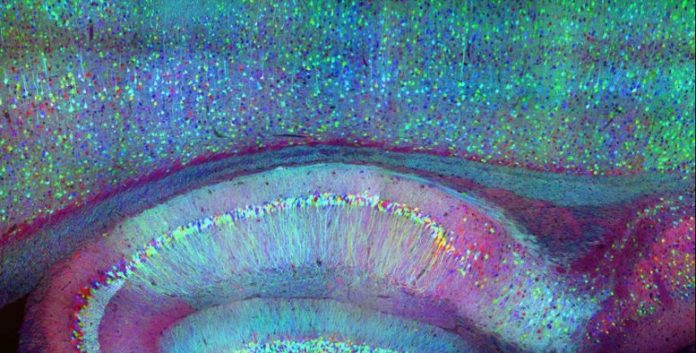
A brand-new method called incorporated neurophotonics might permit scientists to track the activity of all the nerve cells that comprise a specific brain circuit. Credit: Roukes et. al.
A brand-new method called incorporated neurophotonics might permit scientists to track the activity of all the nerve cells that comprise a specific brain circuit.
To deepen their understanding of the brain, neuroscientists should have the ability to map in fantastic information the neural circuits that are accountable for jobs such as processing sensory info or forming brand-new memories. Now, a group of Caltech scientists has actually explained a brand-new method that might permit the activity of all of the thousands to countless nerve cells within a specific brain circuit to be observed in genuine time. The unique technique, gone over in a “Perspective” post released in the journal Neuron on October 14, 2020, has far higher capacity than any existing method, the authors state.
The brand-new method, called “integrated neurophotonics,” utilizes small ranges of optical microchips that can be implanted at any depth inside the brain, in mix with fluorescent molecular press reporters and optogenetic actuators, to optically keep an eye on nerve cells and manage their activity, respectively. The ranges produce microscale beams to promote the genetically customized nerve cells around them and at the exact same time record the activity of these cells, exposing their function. Although the work is presently done just in animal designs, it might one day assistance to unwind circuitry deep inside the human brain, states Michael Roukes, primary private investigator of the paper and Caltech’s Frank J. Roshek Professor of Physics, Applied Physics, and Bioengineering.
“Dense recording at depth—that is the key,” Roukes states. “We will not have the ability to record all of the activity of the brain whenever quickly. But could we concentrate on a few of its essential computational structures within particular brain areas? That’s our inspiration.”
Neuroscientists over the last few years have actually started to utilize optogenetics to study ever-larger groups of nerve cells in design animals consisting of rodents. In optogenetics, nerve cells are genetically crafted to reveal a specific protein marker such as green fluorescent protein (GFP) when delighted by a particular wavelength of light. The existence of GFP triggers the cell to radiance green under fluorescent light, supplying a visual indication of neural activity. By fusing sensing unit particles with these markers, scientists can craft nerve cells that indicate their regional activity by regulating this fluorescence. Optogenetics fixes some issues fundamental in neuroscience research studies that count on implanted electrodes to determine nerve cells’ electrical activity, which usually can dependably determine just a single nerve cell since of all the electrical activity in the brain. Because the brain does not utilize light to interact, optogenetics makes it simpler to track great deals of these neuronal signals.
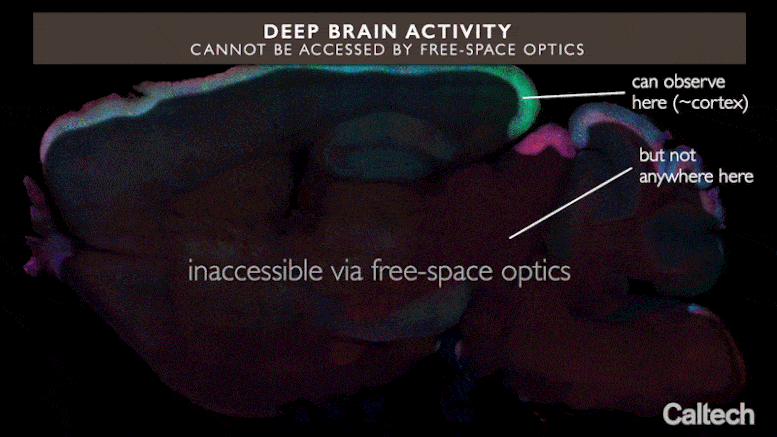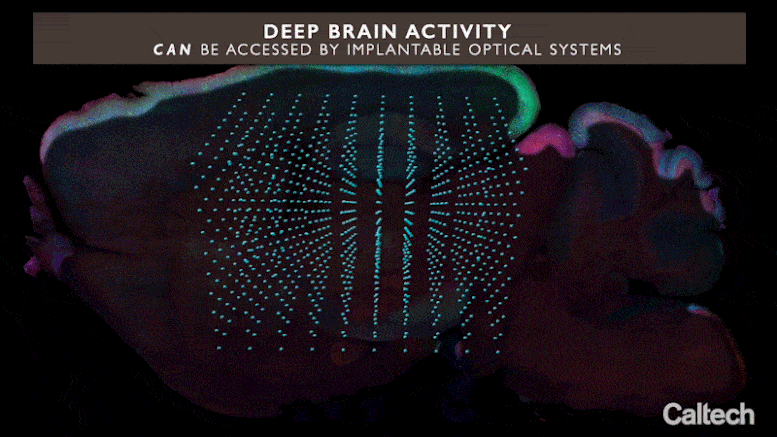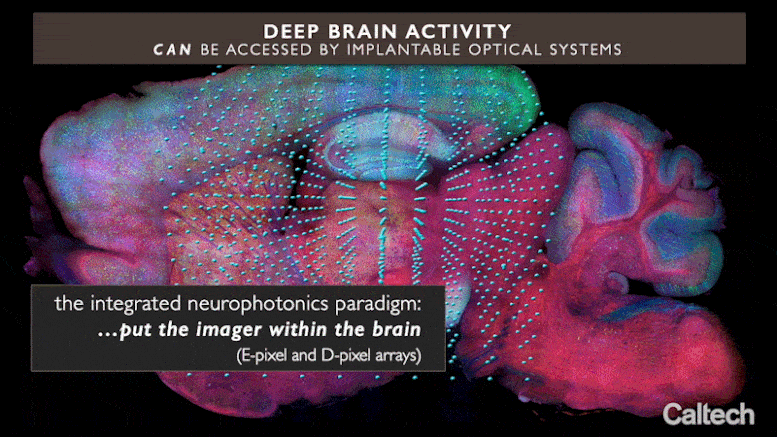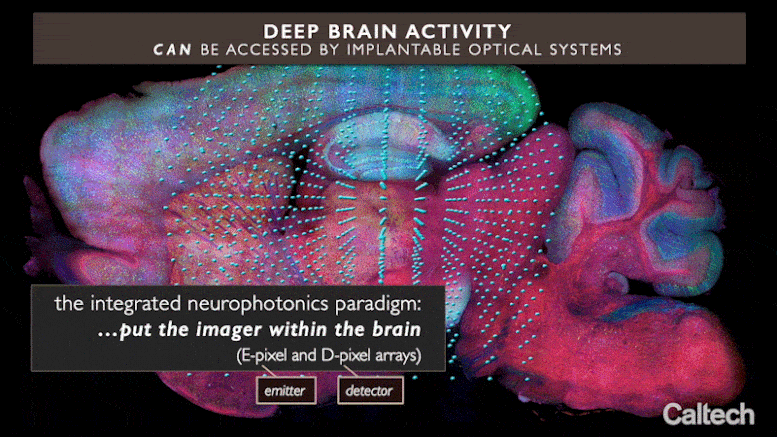
Current optical methods can image nerve cell activity just near the brain’s surface area, however incorporated neurophotonics might open circuits buried deep in the brain. Credit: Roukes et. al.
But existing optogenetic research studies of the brain are constrained by a substantial physical constraint, states Laurent Moreaux, Caltech senior research study researcher and lead author on the paper. Brain tissue spreads light, which suggests that light shone in from outside the brain can take a trip just brief ranges within it. Because of this, just areas less than about 2 millimeters from the brain’s surface area can be taken a look at optically. This is why the best-studied brain circuits are typically easy ones that communicate sensory info, such as the sensory cortex in a mouse—they lie near the surface area. In short, at present, optogenetics techniques cannot easily use insight into circuits situated much deeper in the brain, consisting of those associated with higher-order cognitive or finding out procedures.
Integrated neurophotonics, Roukes and associates state, prevents the issue. In the method, the microscale aspects of a total imaging system are implanted near intricate neural circuits situated deep within the brain, in areas such as the hippocampus (which is associated with memory development), striatum (which manages cognition), and other essential structures in unmatched resolution. Consider the comparable innovation of practical magnetic resonance imaging (fMRI), the scanning method presently utilized to image whole brains. Each voxel, or three-dimension pixel, in an fMRI scan is generally about a cubic millimeter in volume and consists of approximately 100,000 nerve cells. Each voxel, for that reason, represents the typical activity of all of these 100,000 cells.
“The overarching objective of incorporated neurophotonics is to tape what each nerve cell because collection of 100,000 is carrying out in actual time,” Roukes states.
Roukes’s long-lasting objective is to share the sophisticated instrumentation of incorporated neurophotonics to make it possible for multi-institutional cooperations that will leader advanced neuroscience research study with this unique innovation. Previously, he states, this kind of neurotechnology advancement has actually relied mainly upon research study led by a single laboratory or private investigator. Starting in 2011, Roukes dealt with 5 other researchers and the White House Office of Science and Technology Policy to jump-start what eventually ended up being the U.S. BRAIN Initiative (Brain Research through Advancing Innovative Neurotechnologies), released throughout the Obama administration. Their vision was to give neuroscience research study the sort of massive collaborations seen in the physical sciences, as exhibited by hardware advancement jobs such as worldwide telescope cooperations and the LIGO-Virgo cooperation to discover gravitational waves. Now, Roukes states, incorporated neurophotonics opens doors for such instrument-building team effort
“Many of the foundation [for an approach such as ours] have actually existed for a years or more,” he states. “But, until recently, there has just not been the vision, the will, and the funding available to put them all together to realize these powerful new tools for neuroscience.”
The paper explaining this research study is entitled “Integrated Neurophotonics: Toward Dense Volumetric Interrogation of Brain Circuit Activity—at Depth and in Real Time.” Additional Caltech co-authors consist of Wesley D. Sacher, a previous Kavli Nanoscience Institute Prize Postdoctoral Fellow, and previous Caltech postdoctoral scholar Nicole J. Kubat. The work, which included partners from 14 extra organizations, was moneyed by the National Institutes of Health BRAIN Initiative grant, the Defense Advanced Research Projects Agency, the National Science Foundation, and the Kavli Foundation.
Reference: “Integrated Neurophotonics: Toward Dense Volumetric Interrogation of Brain Circuit Activity—at Depth and in Real Time” by Laurent C. Moreaux, Dimitri Yatsenko, Wesley D. Sacher, Jaebin Choi, Changhyuk Lee, Nicole J. Kubat, R. James Cotton, Edward S. Boyden, Michael Z. Lin, Lin Tian, Andreas S. Tolias, Joyce K.S. Poon, Kenneth L. Shepard and Michael L. Roukes, 14 October 2020, Neuron.
DOI: 10.1016/j.neuron.2020.09.043
CaltechAUTHORS: 20201014-111855866