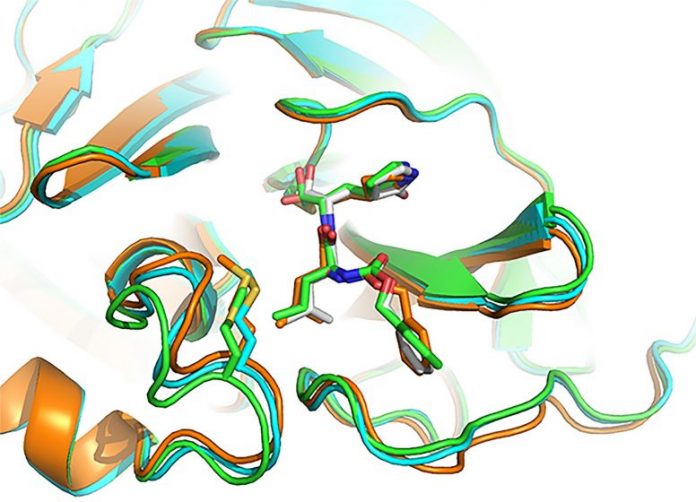
Three setups of active websites where inhibitor GC-376 binds with the COVID-19 infection’s primary protease (drug target Mpro), as portrayed by 3D computer system modeling. Credit: Image produced by Yu Chen, University of South Florida Health, utilizing X-ray crystallography
Four appealing antiviral drug prospects recognized and evaluated by a University of Arizona-University of South Florida group in the preclinical research study.
As the death toll from the COVID-19 pandemic installs, researchers around the world continue their push to establish efficient treatments and a vaccine for the extremely infectious breathing infection.
University of South Florida Health (USF Health) Morsani College of Medicine researchers just recently dealt with coworkers at the University of Arizona College of Pharmacy to determine numerous existing substances that obstruct duplication of the COVID-19 infection (SARS-CoV-2) within human cells grown in the lab. The inhibitors all showed powerful chemical and structural interactions with a viral protein crucial to the infection’s capability to multiply.
The research study group’s drug discovery research study was released on June 15, 2020, in Cell Research, a high-impact Nature journal.

Yu Chen, PhD, an associate teacher of molecular medication at the University of South Florida Health Morsani College of Medicine, has actually turned his with know-how in structure-based drug style towards trying to find brand-new or existing drugs to stop SARS-CoV-2. Credit: © University of South Florida Health
The most appealing drug prospects – consisting of the FDA-approved liver disease C medication boceprevir and an investigational veterinary antiviral drug called GC-376 – target the SARS-CoV-2 primary protease (Mpro), an enzyme that eliminates proteins from a long hair that the infection produces when it attacks a human cell. Without Mpro, the infection cannot duplicate and contaminate brand-new cells. This enzyme had actually currently been verified as an antiviral drug target for the initial SARS and MERS, both genetically comparable to SARS-CoV-2.
“With a rapidly emerging infectious disease like COVID-19, we don’t have time to develop new antiviral drugs from scratch,” stated Yu Chen, PhD, USF Health associate teacher of molecular medication and a coauthor of the Cell Research paper. “A lot of good drug candidates are already out there as a starting point. But, with new information from studies like ours and current technology, we can help design even better (repurposed) drugs much faster.”
Before the pandemic, Dr. Chen used his know-how in structure-based drug style to assist establish inhibitors (drug substances) that target bacterial enzymes triggering resistance to particular typically recommended prescription antibiotics such as penicillin. Now his lab focuses its sophisticated methods, consisting of X-ray crystallography and molecular docking, on trying to find methods to stop SARS-CoV-2.

University of South Florida Health doctoral trainee Michael Sacco dealt with Dr. Chen to figure out the interactions in between antiviral drug prospect GC-376 and COVID-19’s primary protease. Sacco is revealed here taking a look at viral protein crystals under a microscopic lense. Credit: © University of South Florida Health
Mpro represents an appealing target for drug advancement versus COVID-19 since of the enzyme’s important function in the life process of the coronavirus and the lack of a comparable protease in human beings, Dr. Chen stated. Since individuals do not have the enzyme, drugs targeting this protein are less most likely to trigger adverse effects, he discussed.
The 4 leading drug prospects recognized by the University of Arizona-USF Health group as the very best (most powerful and particular) for combating COVID-19 are explained listed below. These inhibitors increased to the top after evaluating more than 50 existing protease substances for prospective repurposing:
- Boceprevir, a drug to deal with Hepatitis C, is the just one of the 4 substances currently authorized by the FDA. Its efficient dosage, security profile, formula and how the body processes the drug (pharmacokinetics) are currently understood, which would considerably accelerate the actions required to get boceprevir to scientific trials for COVID-19, Dr. Chen stated.
- GC-376, an investigational veterinary drug for a lethal pressure of coronavirus in felines, which triggers feline transmittable peritonitis. This representative was the most powerful inhibitor of the Mpro enzyme in biochemical tests, Dr. Chen stated, however prior to human trials might start it would require to be checked in animal designs of SARS-CoV-2. Dr. Chen and his doctoral trainee Michael Sacco identified the X-ray crystal structure of GC-376 bound by Mpro, and defined molecular interactions in between the substance and viral enzyme utilizing 3D computer system modeling.
- Calpain inhibitors II and XII, cysteine inhibitors examined in the past for cancer, neurodegenerative illness and other conditions, likewise revealed strong antiviral activity. Their capability to dually prevent both Mpro and calpain/cathepsin protease recommends these substances might consist of the included advantage of reducing drug resistance, the scientists report.
All 4 substances transcended to other Mpro inhibitors formerly recognized as appropriate to medically assess for dealing with SARS-CoV-2, Dr. Chen stated.
An appealing drug prospect – one that eliminates or hinders the infection without damaging healthy cells — fits comfortably, into the distinct shape of viral protein receptor’s “binding pocket.” GC-376 worked especially well at complying with (matching) the shape of targeted Mpro enzyme binding websites, Dr. Chen stated. Using a lock (binding pocket, or receptor) and secret (drug) example, “GC-376 was by far the key with the best, or tightest, fit,” he included. “Our modeling shows how the inhibitor can mimic the original peptide substrate when it binds to the active site on the surface of the SARS-CoV-2 main protease.”
Instead of promoting the activity of viral enzyme, like the substrate typically does, the inhibitor considerably reduces the activity of the enzyme that assists SARS-CoV-2 make copies of itself.
Visualizing 3-D interactions in between the antiviral substances and the viral protein offers a clearer understanding of how the Mpro complex works and, in the long-lasting, can result in the style of brand-new COVID-19 drugs, Dr. Chen stated. In the meantime, he included, scientists concentrate on getting targeted antiviral treatments to the frontlines quicker by tweaking existing coronavirus drug prospects to enhance their stability and efficiency.
Reference: “Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease” by Chunlong Ma, Michael Dominic Sacco, Brett Hurst, Julia Alma Townsend, Yanmei Hu, Tommy Szeto, Xiujun Zhang, Bart Tarbet, Michael Thomas Marty, Yu Chen and Jun Wang, 15 June 2020, Cell Research.
DOI: 10.1038/s41422-020-0356-z