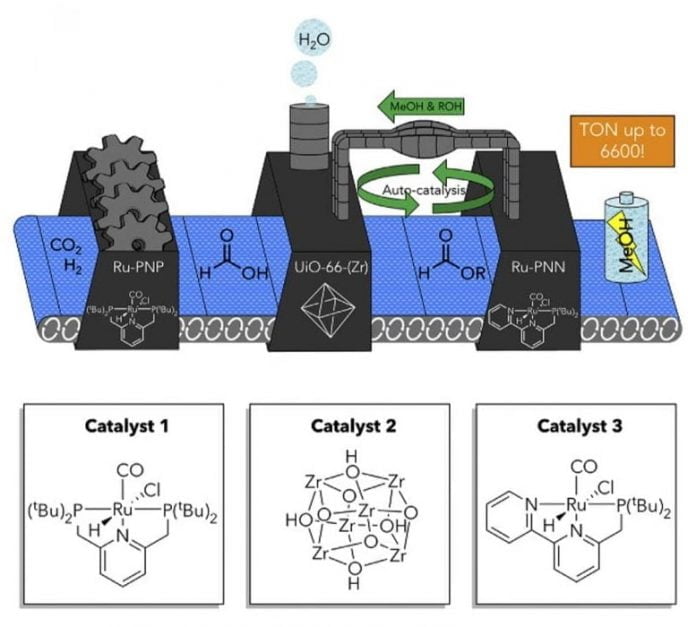
Boston College scientists utilized 3 various drivers in one system to change, in 3 actions, the greenhouse gas co2 to methanol, a liquid fuel that works as an appealing approach for hydrogen storage. The very first driver transforms co2 and hydrogen to formic acid, which is then customized by a 2nd driver to form an ester, integrating an alcohol additive and producing water. The 3rd driver in the system, which is usually incompatible with the very first driver, then transforms this ester to methanol. The group had the ability to perform this multistep response in one response vessel in spite of utilizing 2 incompatible drivers by encapsulating one in a permeable structure that likewise functions as the 2nd driver. Credit: Frank Tsung
Encapsulating several molecular drivers in nanoporous metal-organic structures main to effective change.
Converting co2 to methanol, a possibly sustainable alternative fuel, uses a chance to at the same time form an alternative fuel and reduce co2 emissions.
Inspired by naturally taking place procedures, a group of Boston College chemists utilized a multi-catalyst system to transform co2 to methanol at the most affordable temperature levels reported with high activity and selectivity, the scientists reported in a current online edition of the journal Chem.
The group’s discovery was enabled by setting up several drivers in a single system built within a sponge-like permeable crystalline product referred to as a metal-organic structure, stated Boston College Associate Professors of Chemistry Jeffery Byers and Frank Tsung, lead authors of the report.
Held in location by the sponge, the different drivers operate in consistency. Without seclusion of the catalytically active types in this method, the response did not continue and no item was acquired, they reported.
The group drew its motivation from the biological equipment in cells, which utilize multicomponent chain reaction with terrific effectiveness, Tsung stated.
The group used driver separation through host-guest chemistry — where a “guest” particle is encapsulated in a “host” product to form a brand-new chemical substance — in order to transform co2 to methanol. The method, motivated by the multicomponent catalytic changes in nature, transformed a greenhouse gas to a sustainable fuel while preventing high catalytic need on a single types.
“We accomplished this by encapsulating one or more catalysts in metal-organic framework and applying the resulting host-guest construct in catalysis in tandem with another transition metal complex,” stated Tsung.
The group, that included college student Thomas M. Rayder and undergrad Enric H. Adillon, set out to figure out whether they might establish a method to incorporate incompatible drivers in order to transform co2 to methanol at low temperature level and with high selectivity, stated Byers.
Specifically, they wished to learn if there specify benefits to this method when compared to present cutting-edge systems for shift metal complex-based conversion of co2 to methanol.
“Positioning multiple transition metal complex catalysts at the right position in a system is critical to the reaction turning over,” stated Byers. “At the same time, encapsulating these catalysts allowed for recyclability in the multicomponent catalytic system.”
These residential or commercial properties make the multicomponent driver construct more industrially appropriate, which might lead the way for a carbon-neutral fuel economy, the investigates stated.
In addition to attaining website seclusion by encapsulating the drivers, which caused driver activity and recyclability, the group found an autocatalytic function of the driver that made it possible for the response to be run without the requirement for big quantities of ingredients. Most previous reports for comparable responses utilize big quantities of ingredients, however the group’s method prevents this need and it is the very first to utilize co2 in an energy-related response, Tsung stated.
The group prepares to do additional research study into the modularity of both the encapsulation approach and the metal-organic structures to acquire a much deeper understanding of the multicomponent system and enhance it even more, in addition to gain access to brand-new, undiscovered reactivity through the development of brand-new host-guest constructs, Tsung stated.
Reference: “A Bioinspired Multicomponent Catalytic System for Converting Carbon Dioxide into Methanol Autocatalytically” by Thomas M. Rayder, Enric H. Adillon, Jeffery A. Byers and Chia-Kuang Tsung, 5 May 2020, Chem.
DOI: 10.1016/j.chempr.2020.04.008