New approaches make it possible for researchers at IST Austria to have a look at the innermost of cells: High-resolution pictures of deep-frozen cells reveal structures that formerly might just be rated.
The cells in our body remain in movement. Some move from A to B to recover injuries or battle pathogens. They do so with the assistance of little “feet” at the leading edge of moving cells, so-called lamellipodia. These thin extensions are pressed forward and bind to the surface area while the remainder of the cell is pulled along. Inside these feet is a thick network of interwoven protein threads, called actin filaments, which form the cell’s cytoskeleton. So far, it was uncertain how the Arp2/3 complex, an assembly of 7 proteins main for cell motility, grows off brand-new actin filaments from pre-existing ones and therefore creates thick, branched networks offering the needed protrusive forces to the cell.
Difficult options
Until now, researchers needed to choose when they wished to examine the structure of the Arp2/3 complex: One choice was to study it in seclusion, where the protein complex remains in a non-active conformation and for this reason does not permit understanding of how the network is formed. In order to end up being completely triggered, nevertheless, the Arp2/3 complex requires to be bound to actin filaments. This needs utilizing a technique called electron tomography, which comes at the expense of substantially lower resolution. “Previous electron tomography data of Arp2/3 complexes bound to actin filaments in a test-tube environment was too imprecise, making it impossible to unambiguously tell where the individual elements of the complex must be located,” describes Florian Fäßler, a postdoc in the group of IST Austria teacher Florian Schur.
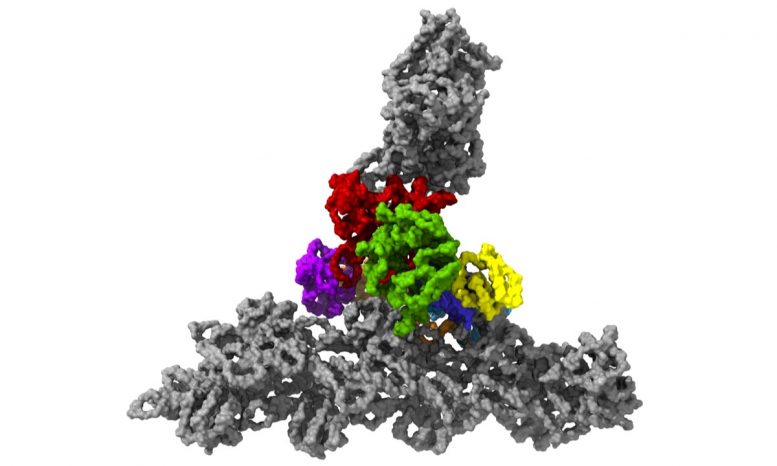
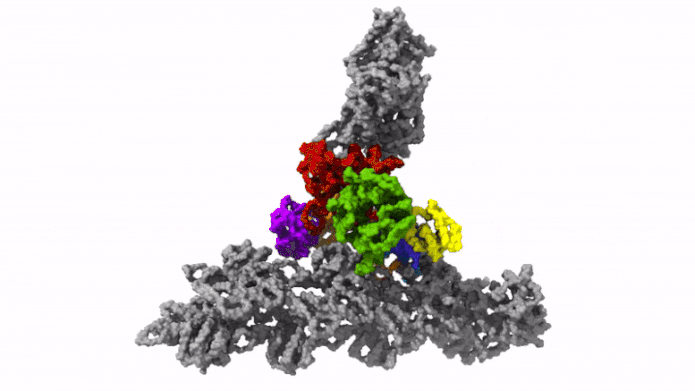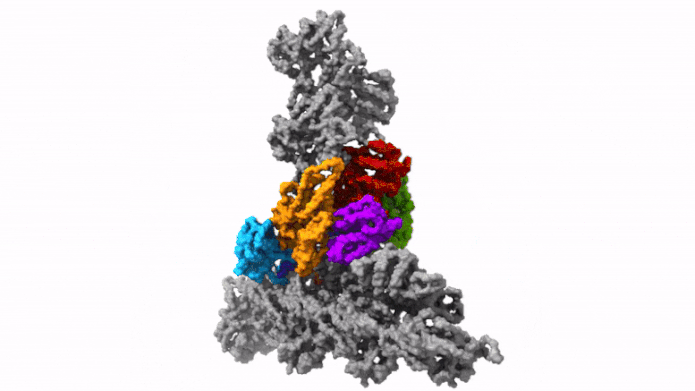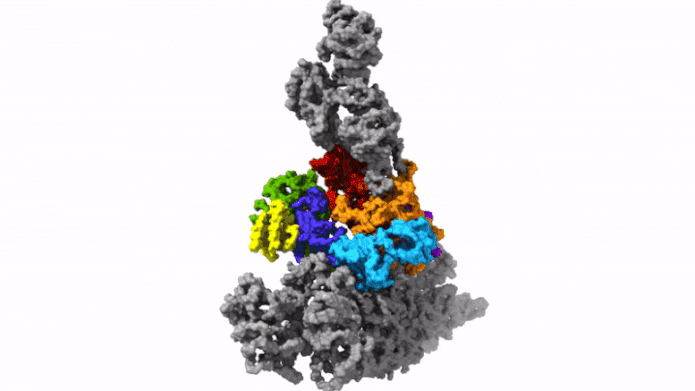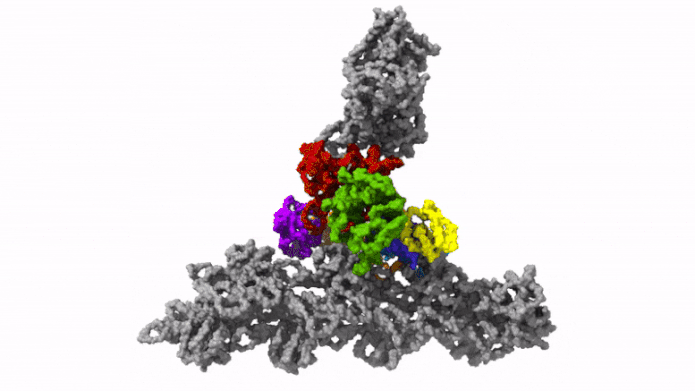
The protein complex Arp2/3 with its 7 subunits (colored) while binding to actin filaments (grey). Credit: © Florian Fäßler, IST Austria
For more than 2 years, he has actually been searching for a method to illustrate the protein complex in its natural surroundings in such a method that the private structures can be examined exactly. Now he has actually been successful. He imaged the complex within lamellipodia of mouse cells in its active actin-bound conformation. “We said to ourselves: Okay, we are going into the cell, where the environment is much more intricate because there is not only the protein complex and actin filaments but all sorts of other things as well. But this was the only way we were able to maintain this network in such a way that we could determine its structure,” states molecular biologist Florian Schur.
Shock-frozen cells
This was enabled by temperature levels of minus 196 degrees Celsius. Within milliseconds, the scientists froze the samples – too rapidly to permit ice crystals to form that would have ruined the cell’s great structures. They then utilized among the most effective cryo-electron microscopic lens offered – and the just one of its kind in Austria – to image cells from various angles utilizing cryo-electron tomography. Doing so, the group gathered enough information for the 3D restoration of over 10,000 Arp2/3 complexes in their active state. Combined with innovative image processing, they then created a 3D design of the Arp2/3 complex at a resolution of less than one nanometer. For contrast: human hair has to do with 50,000 nanometers thick. “We are now able to describe relatively precisely the structure of the protein complex and its subunits and how they form the actin filament network inside the lamellipodium of previously living cells,” states Florian Fäßler. “Five years ago, probably no one would have thought that this could be done,” includes Schur.
To the limitation
Due to the innovative method, the group might refute an earlier design that had actually presumed much bigger location connections in between Arp2/3 complex and actin filaments. However, the researchers validated other elements of how this complex is managed and forms brand-new actin filaments. With this understanding, other researchers can now much better comprehend this essential protein complex’s guideline and activity in its numerous functions beyond cell motility and the advancement of illness. “What we have done is to go as far as is currently possible with such complex samples in terms of methodology and resolution. With the current resolution, we have gained new biological insights, but it was also a methodological advance to show: It is possible,” Schur states enthusiastically. Florian Fäßler now wishes to enhance the technique even further to imagine other proteins and check out how far the technique permits us to see inside a cell. “We are just starting to realize the full potential of cryo-electron tomography,” states Schur.
Credit: “Cryo-electron tomography structure of Arp2/3 complex in cells reveals new insights into the branch junction” by Florian Fäßler, Georgi Dimchev, Victor-Valentin Hodirnau, William Wan and Florian K. M. Schur, 22 December 2020, Nature Communications.
DOI: 10.1038/s41467-020-20286-x
Funding: Austrian Science Fund (FWF), Georgi Dimchev, Florian Schur.