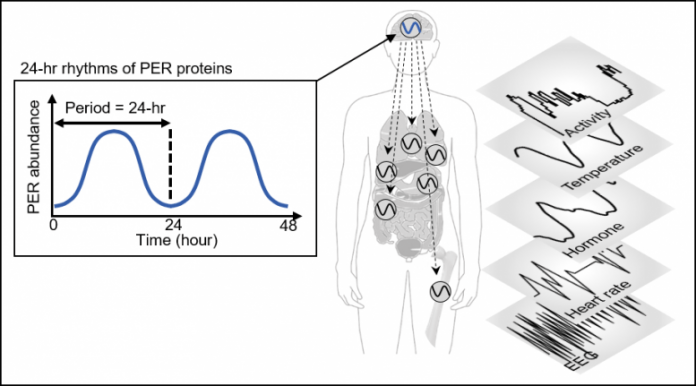
Figure 1. PER abundance oscillates in a circadian method by an autoregulatory detrimental suggestions loop. This oscillation allows strong timekeeping of circadian-timed physiological and behavioral processes together with sleep. Credit: KAIST
KAIST mathematicians and their collaborators at Florida State University have recognized the precept of how growing old and ailments like dementia and weight problems trigger sleep problems. A mixture of mathematical modeling and experiments demonstrated that the cytoplasmic congestion brought on by growing old, dementia, and/or weight problems disrupts the circadian rhythms within the human physique and results in irregular sleep-wake cycles. This discovering suggests new therapy methods for addressing unstable sleep-wake cycles.
Human our bodies modify sleep schedules in accordance with the ‘circadian rhythms,’ that are regulated by our time conserving system, the ‘circadian clock.’ This clock tells our physique when to relaxation by producing the 24-hour rhythms of a protein known as PERIOD (PER) (See Figure 1).
The quantity of the PER protein will increase for half of the day after which decreases for the remaining half. The precept is that the PER protein accumulating within the cytoplasm for a number of hours enters the cell nucleus unexpectedly, hindering the transcription of PER genes and thereby lowering the quantity of PER.
However, it has remained a thriller how 1000’s of PER molecules can concurrently enter into the nucleus in a fancy cell surroundings the place quite a lot of supplies co-exist and might intrude with the movement of PER. This can be like discovering a means for 1000’s of staff from throughout New York City to enter an workplace constructing on the similar time day-after-day.
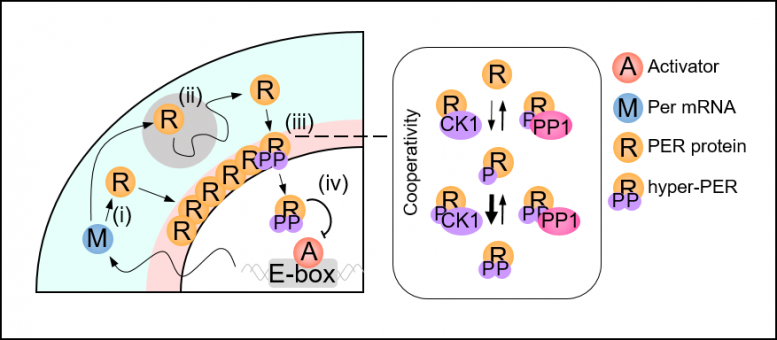
Figure 2. Spatial Stochastic mannequin of the circadian clock. After Per mRNA, M, is translated to protein, R, within the cytoplasm (i), PER transits towards the perinucleus, previous obstacles whereas being hypophosphorylated (ii; grey circle). The gathered PER within the perinucleus is hyperphosphorylated in a cooperative method (iii). Then it enters the nucleus and inhibits the transcriptional exercise of the activator, A (iv). Credit: KAIST
A bunch of researchers led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences solved the thriller by creating a spatiotemporal and probabilistic mannequin that describes the movement of PER molecules in a cell surroundings.
This examine was carried out in collaboration with Professor Choogon Lee’s group from Florida State University, the place the experiments had been carried out, and the outcomes had been printed within the Proceedings of the National Academy of Sciences (PNAS) final month.
The joint analysis workforce’s spatial stochastic mannequin (See Figure 2) described the movement of PER molecules in cells and demonstrated that the PER molecule ought to be sufficiently condensed across the cell nucleus to be phosphorylated concurrently and enter the nucleus collectively (See Figure 3 Left). Thanks to this phosphorylation synchronization change, 1000’s of PER molecules can enter the nucleus on the similar time day-after-day and keep secure circadian rhythms.
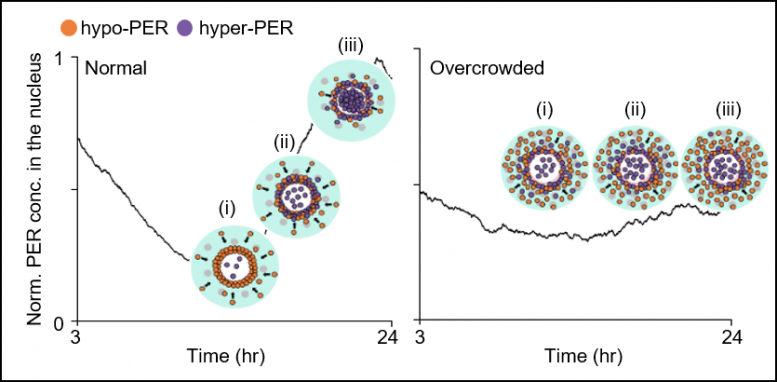
Figure 3. In regular cell (left), the cytoplasmic flux over a number of hours will increase PER abundance within the perinucleus (pink area in Figure 2) in contrast with the peripheral cytoplasm (cyan area in Figure 2) (i). This induces a pointy switch-like hyperphosphorylation within the perinucleus as a result of cooperativity (ii), adopted by synchronous nuclear entry inside a slim time window (iii). When a cell is overcrowded (proper), the cytoplasmic flux is hindered, and thus PER doesn’t accumulate in the identical gradient as within the regular cell (i). This disables the sharp switch-like PER hyperphosphorylation and nuclear entry (ii and iii). Credit: KAIST
However, when growing old and/or ailments together with dementia and weight problems trigger the cytoplasm to turn into congested with elevated cytoplasmic obstacles corresponding to protein aggregates and fats vacuoles, it hinders the well timed condensation of PER molecules across the cell nucleus (See Figure 3 Right). As a outcome, the phosphorylation synchronization change doesn’t work and PER proteins enter into the nucleus at irregular occasions, making the circadian rhythms and sleep-wake cycles unstable, the examine revealed.
Professor Kim mentioned, “As a mathematician, I am excited to help enable the advancement of new treatment strategies that can improve the lives of so many patients who suffer from irregular sleep-wake cycles. Taking these findings as an opportunity, I hope to see more active interchanges of ideas and collaboration between mathematical and biological sciences.”
Reference: “Wake-sleep cycles are severely disrupted by diseases affecting cytoplasmic homeostasis” by Stephen Beesley, Dae Wook Kim, Matthew D’Alessandro, Yuanhu Jin, Kwangjun Lee, Hyunjeong Joo, Yang Young, Robert J. Tomko Jr, John Faulkner, Joshua Gamsby, Jae Kyoung Kim and Choogon Lee, 26 October 2020, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2003524117
This work was supported by the National Institutes of Health and the National Science Foundation within the US, and the International Human Frontiers Science Program Organization and the National Research Foundation of Korea.