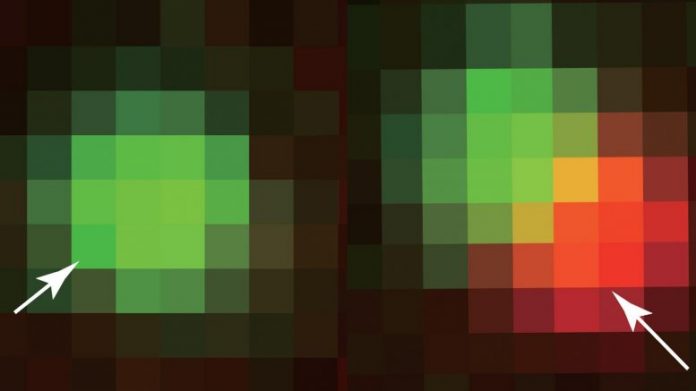
Princeton scientists have actually established a structure to craft the protein beads that arrange essential functions inside a cell. Key to their findings was the theory of bubble development, adjusted from classical products science. In the above, a crafted protein piece (green) “seeds” the development of a protein bead (red), the basis for higher-order organelles such as the nucleolus. The brand-new work marks a seismic shift in researchers’ capability to control cells. Credit: Image thanks to the scientists
Theory of Bubbles Lifts Cell Biology Into a New, More Quantitative Era
A research study released on September 22, 2021, in the journal Nature information how a recognized physics theory governing bubble and bead development caused a brand-new understanding of the concepts arranging the contents of living cells. The work marks a seismic shift in scientists’ capability both to comprehend and manage the complicated soft products within our cells.
“This approach is common in materials science, but we’ve adapted it to do something unprecedented in cells,” stated primary private investigator Clifford Brangwynne, the June K. Wu ’92 Professor in Engineering and director of the Princeton Bioengineering Initiative.
The existing work follows Brangwynne’s discovery more than a years ago that cellular proteins arrange into liquid structures inside the cell. That insight triggered a brand-new discipline taking a look at how parts of cells form similar to oil drops coalescing in water. Scientists have actually puzzled since over the precise information of how those structures put together. But it’s a difficult thing to determine the squishy characteristics of private particles inside a cell, where mystical, overlapping procedures roil chaotically as minute structures form and liquify a thousand times per second.
Postdoctoral scientist Shunsuke Shimobayashi had actually studied soft matter physics at the Kyoto University and questioned whether his background dealing with natural substances called lipids may brighten anything fascinating about the issue. If protein particles condense out of their environments the method oil separates from water, possibly the mathematics that explained the primary steps because procedure, called nucleation, would show helpful in proteins also.
Shimobayashi relied on classical nucleation theory, a pillar of products science. Its formulas had actually powered a few of the most extensive technological improvements of the 20 th century, from the environment designs that initially exposed worldwide warming to the fertilizers that assisted raise billions of individuals out of hunger.
He was likewise acutely familiar with a vital difference: those formulas explain easy, inanimate systems, however the within a cell remains in chaos. “It’s a much more complex material environment for biomolecules,” Shimobayashi stated. To tackle this intricacy, the group broadened to consist of theorists Pierre Ronceray, a previous fellow at the Princeton Center for Theoretical Science, and Mikko Haataja, teacher of mechanical and aerospace engineering whose previous theoretical and computational deal with the Brangwynne laboratory had actually caused crucial insights in associated research studies. The scientists removed the theory to its 2 essential criteria, adjusting it to attempt to comprehend how the procedure may operate in cells. Then to check the theory, Shimobayashi relied on an innovative protein tool established in Brangwynne’s laboratory in 2018 that supplied a perfect, streamlined system that imitates how the procedure happens naturally in cells. Putting them together, the outcomes came as something of a shock.
When Shimobayashi attempted to cause the beads to seed immediately, the system stopped working. But when he seeded the beads more gradually, they nucleated at exactly specified places, in a manner that lined up completely with his adjusted theory. He had actually anticipated how, where and when the protein beads formed with what Brangwynne called “amazing precision“
The group next reversed to the unpleasant intricacy of native cell structures, now working together with another Brangwynne laboratory postdoc, David Sanders, a professional on internal cell structures called tension granules. When they represented all the procedures that act upon protein concentrations, they discovered that the theory worked simply as well for tension granules and other condensates. They had actually measured the molecule-by-molecule assembly of proteins into the complex liquid structures that control life’s many fundamental regimens. Not just do these structures look and imitate oil in water, Shimobayashi stated, they likewise form beads in the very same fundamental nucleation patterns, clustering around minute variations in their environment at rates that can be anticipated with the very same quantitative accuracy as other type of products.
With that predictive power comes a sped up engineering capability, according toBrangwynne He thinks measuring biomolecular procedures and establishing predictive designs in the mold of physics will cause a world in which we no longer view passively as our enjoyed ones catch illness like Alzheimer’s
“We first have to understand how it works, with quantitative mathematical frameworks that are the bedrock of society’s engineering marvels. And then we can take the next steps, to manipulate biological systems with greater control,” Brangwynne stated. “We need to be able to turn the knobs.”
Reference: “Nucleation landscape of biomolecular condensates” by Shunsuke F. Shimobayashi, Pierre Ronceray, David W. Sanders, Mikko P. Haataja and Clifford P. Brangwynne, 22 September 2021, Nature
DOI: 10.1038/ s41586-021-03905 -5
This work, entitled “Nucleation landscape of biomolecular condensates,” was supported in part by the Howard Hughes Medical Institute, a Focused Research Team Award from Princeton’s School of Engineering and Applied Science, the National Institutes of Health and the Princeton Center for Complex Materials., Air Force Office of Scientific Research, and the Princeton Center for Complex Materials.