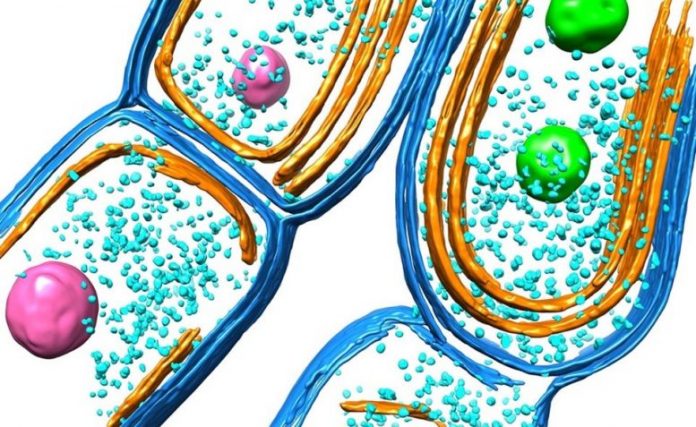
Illustration of the cyanobacterial thylakoid membrane. Credit: Luning Liu et al.
A brand-new research study performed by the scientists at the University of Liverpool exposes how the ancient photosynthetic organisms – cyanobacteria – develop their photosynthetic equipment and arrange their photosynthetic membrane architecture for the effective capture of solar light and energy transduction.
Oxygenic photosynthesis, performed by plants, algae, and cyanobacteria, produces energy and oxygen for life on Earth and is perhaps the most essential biological procedure. Cyanobacteria are amongst the earliest phototrophs that can carry out oxygenic photosynthesis and make considerable contributions to the Earth’s environment and main production.
Light-reliant photosynthetic responses are carried out by a set of photosynthetic complexes and particles accommodated in the specialized cell membranes, called thylakoid membranes. While some research studies have actually reported the structures of photosynthetic complexes and how they carry out photosynthesis, scientists still had little understanding about how native thylakoid membranes are developed and more established to end up being a practical entity in cyanobacterial cells.
The research study group, led by Professor Luning Liu from the University’s Institute of Systems, Molecular and Integrative Biology, established a technique to manage the development of thylakoid membranes throughout cell development and utilized advanced proteomics and tiny imaging to define the step-by-step maturation procedure of thylakoid membranes. Their outcomes are released in the journal Nature Communications.
“We are really thrilled about the findings,” stated Professor Liu. “Our research draws a picture about how phototrophs generate and then develop their photosynthetic membranes, and how different photosynthetic components are incorporated and located in the thylakoid membrane to perform efficient photosynthesis – a long-standing question in this field.”
The very first author of the research study, Dr. Tuomas Huokko, stated: “We find that the newly synthesized thylakoid membranes emerge between the peripheral cell membrane, termed the plasma membrane, and the pre-existing thylakoid layer. By detecting the protein compositions and photosynthetic activities during the thylakoid development process, we also find that photosynthetic proteins are well controlled in space and time to evolve and assemble into the thylakoid membranes.”
The brand-new research study reveals that the cyanobacterial thylakoid membrane is a genuinely vibrant biological system and can adjust quickly to ecological modifications throughout bacterial development. In thylakoids, photosynthetic proteins can diffuse from one position to another and form practical “protein islands” to interact for high photosynthetic effectiveness.
“Since cyanobacteria perform plant-like photosynthesis, the knowledge gained from cyanobacteria thylakoid membranes can be extended to plant thylakoids,” included Professor Liu. “Understanding how the natural photosynthetic machinery is evolved and regulated in phototrophs is vital for tuning and enhancing photosynthetic performance. This offers solutions to sustainably improve crop plant photosynthesis and yields, in the context of climate change and growing population. Our research may also benefit the bioinspired design and generation of artificial photosynthetic devices for efficient electron transfer and bioenergy production.”
Reference: “Probing the biogenesis pathway and dynamics of thylakoid membranes” by Tuomas Huokko, Tao Ni, Gregory F. Dykes, Deborah M. Simpson, Philip Brownridge, Fabian D. Conradi, Robert J. Beynon, Peter J. Nixon, Conrad W. Mullineaux, Peijun Zhang and Lu-Ning Liu, 9 June 2021, Nature Communications.
DOI: 10.1038/s41467-021-23680-1
The research study was performed in cooperation with the University’s Centre for Proteome Research, Centre for Cell Imaging, and Biomedical Electron Microscopy Unit, along with with scientists from University of Oxford, Queen Mary University of London, and Imperial College London. The research study was moneyed by the BBSRC, Royal Society, Wellcome Trust, and Leverhulme Trust.