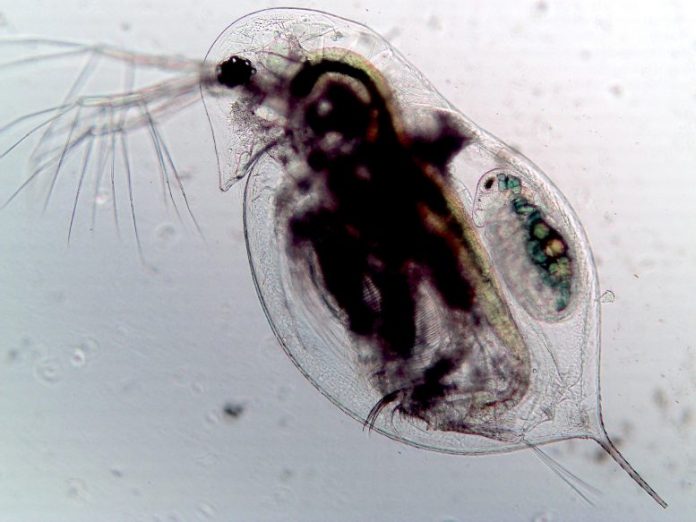
A zooplankton (Daphnia dentifera) contaminated by the fungal parasite Metschnikowia bicuspidate. The tiny fungal spores filling the body as noticeable as black fuzzy areas. Credit: Tara Stewart Merrill
Whether it’s plankton exposed to parasites or individuals exposed to pathogens, a host’s preliminary immune reaction plays an essential function in figuring out whether infection happens and to what degree it spreads out within a population, brand-new CU Boulder research study recommends.
The findings, released on May 13, 2021, in The American Naturalist, offer important insight for understanding and avoiding the transmission of illness within and in between animal types. From parasitic flatworms sent by snails into people in establishing countries, to zoonotic spillover occasions from mammals and pests to people—which have actually triggered worldwide pandemics like COVID-19 and West Nile infection—a contaminated animal’s immune reaction is a crucial variable to think about in determining what occurs next.
“One of the biggest patterns that we’re seeing in disease ecology and epidemiology is the fact that not all hosts are equal,” stated Tara Stewart Merrill, lead author of the paper and a postdoctoral fellow in ecology. “In infectious disease research, we want to build host immunity into our understanding of how disease spreads.”
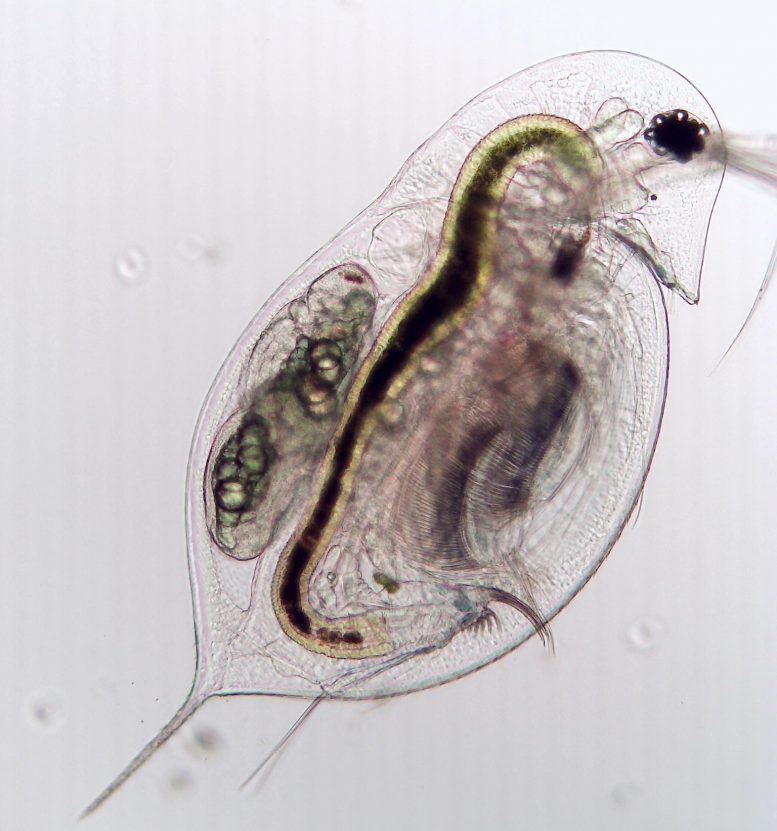
A zooplankton (Daphnia dentifera) not contaminated by fungal parasite Metschnikowia bicuspidate. Credit: Tara Stewart Merrill
Invertebrates prevail vectors for illness, which indicates they can send transmittable pathogens in between people or from animals to people. Vector-borne illness, like malaria, represent nearly 20% of all transmittable illness around the world and are accountable for more than 700,000 deaths each year.
Yet epidemiological research studies have actually seldom thought about invertebrate resistance and healing in animals that are vectors for human illness. They presume that when exposed to a pathogen, the invertebrate host will end up being contaminated.
But what if it was possible for invertebrates to eliminate off these illness, and break the link in the chain that passes them on to people?
While observing a small types of zooplankton (Daphnia dentifera) throughout its lifecycle and direct exposure to a fungal parasite (Metschnikowia bicuspidata), the scientists saw this capacity in action. Some of the plankton were proficient at stopping fungal spores from entering their bodies, and others cleared the infection within a restricted window of time after consuming the spores.
“Our results show that there are several defenses that invertebrates can use to reduce the likelihood of infection, and that we really need to understand those immune defenses to understand infection patterns,” stated Stewart Merrill.

Tara Stewart Merrill. Credit: Loren Merrill
Unexpected healing
Stewart Merrill began this operate in her very first year as a doctoral trainee at the University of Illinois, studying this little plankton and its collection of defenses. It’s a gruesome procedure if the plankton stops working to fend off the parasite: Its fungal spores assault the plankton’s gut, fill its body and grow till they are launched when the host lastly passes away.
But she discovered something that had actually not been tape-recorded prior to: Some of the doomed plankton recuperated. Several years later on, she has actually discovered that when confronted with similar levels of direct exposure, the success or failure of these infections depends upon the strength of the host’s internal defenses throughout this early minimal window of chance.
Based on their observations of these private results, the scientists established a basic probabilistic design for determining host resistance that can be used throughout wildlife systems, with essential applications for illness sent to people by invertebrates.
“When immune responses are good, they act as a filter that reduces transmission,” stated Stewart Merrill. “But any environmental change that degrades immunity can actually amplify transmission, because it will let all of that exposure go through and ultimately become infectious.”
It’s a design that can likewise use to COVID-19, as research study from CU Boulder has actually revealed that not all hosts are the exact same in sending the coronavirus, and direct exposure does not straight identify infection.
COVID-19 is likewise thought to be the outcome of a zoonotic spillover, an infection that moved from animals into individuals, and comparable probabilistic designs might be beneficial in anticipating the event and spread of future spillover occasions, stated Stewart Merrill.
Understanding avoidance of infection
Stewart Merrill hopes that a much better understanding of infections in a basic animal like plankton can be used more broadly to invertebrates that matter for human health.
In Africa, Southeast Asia, in addition to South and Central America, 200 million individuals experience infections triggered by schistosomes—invertebrates more frequently referred to as parasitic flatworms. They trigger health problem and death, and considerable financial and public health effects, a lot so that the World Health Organization considers them the second-most socioeconomically ravaging parasitic illness after malaria.
They’re simply among numerous ignored tropical illness sent to individuals by invertebrate hosts such as snails, mosquitoes and biting flies. These illness contaminate a big part of a population however happen in locations with low levels of sanitation that don’t have the financial resources to resolve those illness, stated Stewart Merrill.
Schistosomes reside in freshwater environments that individuals utilize for their drinking water, laundry and bathing. So despite the fact that there are treatments, the next day an individual can quickly get reinfected simply by accessing the water they require. By much better understanding how the flatworms themselves catch or battle infection, researchers like Stewart Merrill assist us get closer to stopping the chain of transmission into people.
“We really need to work on understanding prevention of infection, and what that risk is in those aquatic systems, rather than just cures for infection,” she stated.
The great news is we can gain from the exact same invertebrates which contaminate us. In invertebrate hosts that suffer or pass away from their infections, there is a great reward to find out how to construct an immune reaction and battle it off. Some snails have actually even revealed the capability to maintain an immunological memory: If they get contaminated when and endure, then they may never ever get contaminated once again.
“If we can better understand how the environment shapes those defenses, we could predict into the future how environmental changes might amplify or suppress risk of transmission to people,” stated Stewart Merrill.
Reference: “Host controls of within-host disease dynamics: insight from an invertebrate system” by Tara Stewart Merrill, Zoi Rapti and Carla E Cáceres, 13 May 2021, The American Naturalist.
DOI: 10.1086/715355
Additional authors on this paper consist of Zoi Rapti and Carla Cáceres at the University of Illinois.