Artistic making of a growth growing within a colon, leading to regional changes of resident microbiota. Credit: Sarah Field Sonnenberg
An immune cell subset called inherent lymphoid cells (ILC3s) secures versus colorectal cancer, in part by assisting to keep a healthy discussion in between the body immune system and gut microorganisms, according to a brand-new research study led by scientists at Weill Cornell Medicine and NewYork-Presbyterian The finding unlocks to brand-new techniques for treating this kind of cancer.
The scientists, who released their findings in the journal Cell, revealed that ILC3s tend to be significantly lowered and functionally modified in individuals with colorectal cancer. Further, they show that experimentally interfering with the functions of ILC3s in mice causes aggressive colon cancer and significantly decreases the efficiency of cancer immunotherapies.
What Is Colorectal Cancer?
Colorectal cancer is an illness in which cells in the colon or anus outgrow control. Sometimes it is called colon cancer. The colon is the big intestinal tract or big bowel. The anus is the passage that links the colon to the rectum.
Colorectal cancer is the 4th most typical cancer in the United States, with about 150,000 brand-new cases each year and about 50,000 deaths. While early detection of these cancers or precancerous polyps with screening colonoscopies is really efficient, treatments for sophisticated colorectal growths stay a significant obstacle with restricted healing alternatives. Oncologists are especially worried about the relative resistance of these growths to immunotherapies– treatments that work well versus some other cancers by increasing the body immune system’s capability to attack deadly cells.
“These findings suggest new possibilities for the clinical approach to colorectal cancer, and also help explain why this type of cancer often fails to respond to immunotherapies,” stated senior authorDr Gregory Sonnenberg, an associate teacher of microbiology and immunology in medication in the Division of Gastroenterology and Hepatology and a member of the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine.
One element affecting the resistance to immunotherapies might be the gut microbiome, the population of germs and other microbial types that live in the intestinal tracts and generally help food digestion, assistance different metabolic functions and contribute in managing the body immune system. Colorectal cancer is connected with persistent gut swelling and a significant interruption of the typical microbiome. Further, current research studies recommend that clients’ microbiomes play a crucial function in managing the result of cancer immunotherapies and might discuss why some clients’ cancers do, or do not, react well to treatment.
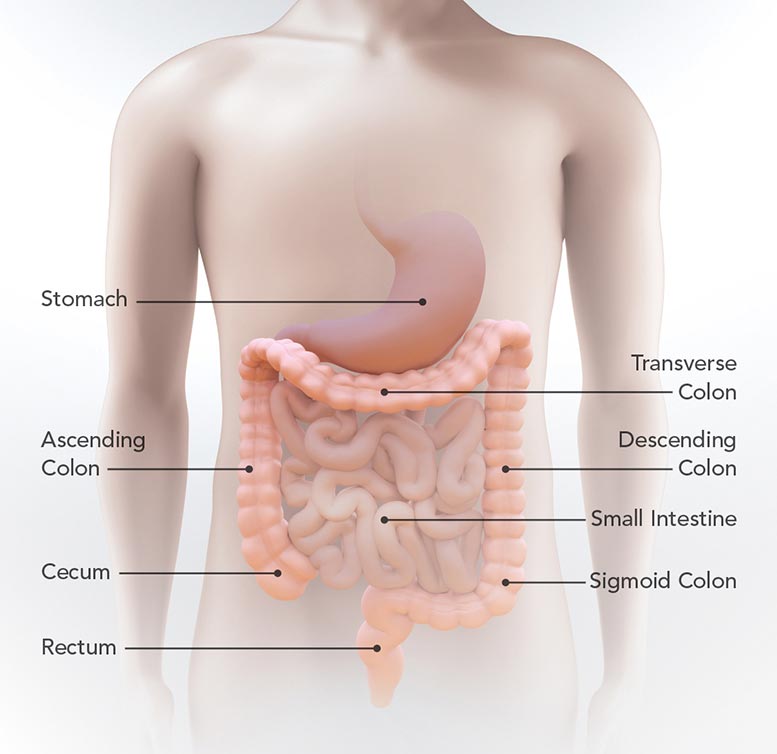
This diagram reveals the place of the stomach, little intestinal tract, cecum, rising colon, transverse colon, coming down colon, sigmoid colon, and anus. Credit: CDC
In the brand-new research study,Dr Sonnenberg and associates, consisting of lead authorDr Jeremy Goc, a research study partner inDr Sonnenberg’s lab, analyzed the function of ILC3s, which live in the intestinal tracts and are understood to assist moderate the relationship in between the body immune system and gut microorganisms.
Group 3 inherent lymphoid cells generally play a crucial function in preserving a healthy discussion in between the microbiome and the immune environment in the lower gut. In close cooperation withDr Manish Shah, the Bartlett Family Professor of Gastrointestinal Oncology, director of the Gastrointestinal Oncology Program in the Division of Hematology and Medical Oncology, and member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine, the research study group evaluated colorectal growths and pre-cancerous polyps from people and mice. They discovered that ILC3s from malignant tissues were reasonably diminished as compared to healthy tissues and were more essentially modified in their functions.
“This is an exciting finding that could have broad implications for our understanding of the pathways that control the pathogenesis, progression and therapeutic responsiveness of gastrointestinal malignancies,” stated research study co-authorDr Shah, who is likewise chief of the Solid Tumor Oncology Service and co-director of the Center for Advanced Digestive Care at NewYork-Presbyterian/Weill Cornell Medical Center.
Among this loss of typical ILC3 activity in the gut, the authors even more observed that the capability of ILC3s to manage a particular immune cell subset called T cells was substantially interrupted. This interruption of the discussion in between ILC3s and T cells even more resulted in an increase in swelling in the gut that consequently customizes the gut microbiome. These gut microorganism modifications in turn cause a decline in the levels of T cells that are proficient at battling growths.
Those cumulative results have significant effects on growth advancement, the scientists revealed. In mice that establish colon cancers, obstructing ILC3 signaling resulted in the development of unusually intrusive and more aggressive growths with bad result. And when colon growths were implanted in mice with obstructed ILC3 signaling, the growths were reasonably unresponsive to a cancer immunotherapy called anti-PD-1 checkpoint blockade– whereas the exact same sort of growth, implanted in mice with typical ILC3 signaling, reacted well to the treatment.
Finally, in biopsied colorectal tissues from clients with inflammatory bowel illness (IBD), the scientists discovered ILC3-related problems comparable to those in colorectal cancer clients. Transplanting the microorganisms from IBD clients into mice gave resistance to treatment– whereas mice transplanted with microorganisms from healthy human donors still reacted well to anti-PD-1 checkpoint blockade.
“Better understanding the contribution of the microbiome to cancer development and treatment responsiveness could revolutionize patient management strategies. This study illuminates a mechanism of therapy resistance driven by dysregulation of the microbiome that has been unappreciated until now,”Dr Goc stated. “It suggests, for example, that one day we could sample gut microbiota to predict tumor progression and responsiveness to immunotherapy—and even use healthy microbiota to improve treatment responsiveness.”
The scientists are now working to determine the types of gut germs that are most helpful in this regard. This research study is supported in part by an unique financing system from the Cancer Research Institute that was approved toDr Sonnenberg in 2019, the inaugural year of the program.
Reference: “Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer” by Jeremy Goc, Mengze Lv, Nicholas J. Bessman, Anne-Laure Flamar, Sheena Sahota, Hiroaki Suzuki, Fei Teng, Gregory G. Putzel, JRI Live Cell Bank, Gerard Eberl, David R. Withers, Janelle C. Arthur, Manish A. Shah and
Gregory F. Sonnenberg, 17 August 2021, Cell
DOI: 10.1016/ j.cell.202107029
Research in the Sonnenberg Laboratory is likewise supported by the National Institutes of Health (R01 AI143842, R01 AI123368, R01 AI145989, U01 AI095608, R21 CA249274 and R01 AI162936), an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, a Wade F.B. Thompson/Cancer Research Institute (CRI) CLIP Investigator grant, a CRI Lloyd J. Old STAR Award, the Meyer Cancer Center Collaborative Research Initiative, The Dalton Family Foundation, and Linda and GlennGreenberg Jeremy Goc is supported by fellowships from the Crohn’s and Colitis Foundation (519428) and the PhilippeFoundation Greg Sonnenberg is a CRI Lloyd J. Old STAR. Gregory F. Sonnenberg was formerly on a clinical board of advisers and owned stock in Celsius Therapeutics Inc within the past 12 months.





