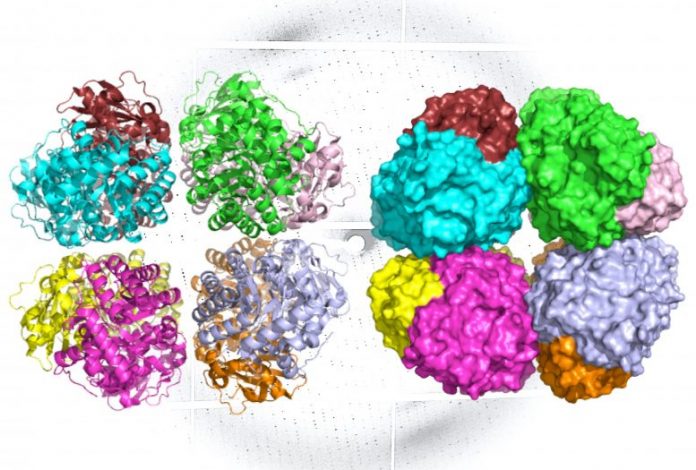
A ribbon diagram (L) and molecular surface area representation (R) of carbon-fixing type I’ rubisco, revealing 8 molecular subunits without the little subunits. An x-ray diffraction pattern of the enzyme, likewise created by the research study group, remains in the background. Credit: Henrique Pereira/Berkeley Lab
A group of researchers has actually found an ancient type of rubisco, the most plentiful enzyme on Earth and vital to life as we understand it.
Found in formerly unidentified ecological microorganisms, the freshly determined rubisco offers insight into the advancement of the photosynthetic organisms that underlie the world’s food cycle.
“Rubisco is the main motorist for producing food, so it can take CO2 from the environment and repair that into sugar for plants and other photosynthetic organisms to utilize,” stated Doug Banda, a postdoctoral scholar in the laboratory of Patrick Shih, a UC Davis assistant teacher and the director of Plant Biosystems Design at the Joint BioEnergy Institute (JBEI), which is handled by Lawrence Berkeley National Laboratory (Berkeley Lab). “It is also one of the oldest carbon-fixing enzymes on the planet.”
Form I rubisco, which is discovered in plants, algae, and cyanobacteria, has a deep evolutionary history with the world, returning almost 2.4 billion years to the Great Oxygenation Event, when cyanobacteria actually changed the Earth’s environment by presenting oxygen to it through photosynthesis. Rubisco’s function in this fundamental occasion makes it a crucial focus of researchers studying the advancement of life, in addition to researchers looking for to establish bio-based fuels and renewable resource innovations.
In a research study appearing in Nature Plants, Banda and scientists from UC Davis, UC Berkeley, and Berkeley Lab report the discovery and characterization of a formerly undescribed family tree of type I rubisco – one that the scientists presume diverged from type I rubisco prior to the advancement of cyanobacteria.
Found through metagenomic analysis of ecological samples and manufactured in a laboratory, the brand-new family tree, called type I’ rubisco, offers scientists brand-new insights into the structural advancement of type I rubisco, possibly supplying ideas regarding how this enzyme altered the world.
“This could’ve been what a rubisco looked like before the rise of oxygen more than 2.4 billion years ago,” stated Shih, keeping in mind that the type I’ rubisco offers researchers with a window into how ancient microorganisms may’ve repaired carbon prior to the increase of cyanobacteria and the type I rubisco.
An unnoticeable world
Form I rubisco is a hexadecamer, suggesting it’s developed from 8 core, big molecular subunits with 8 little subunits set down on top and bottom. Each piece of this protein’s structure is important to photosynthesis, and hence the carbon fixation procedure.
Other practical types of rubisco exist in germs and bacteria of the Archaea domain. These variations can be found in various sizes and shapes, and all carry out the exact same action of photosynthesis. However, type I rubisco is accountable for the large bulk of carbon fixation on Earth.
Study co-author and partner Professor Jill Banfield, of UC Berkeley’s Earth and Planetary Sciences Department, exposed type I’ rubisco after carrying out metagenomic analyses on groundwater samples. Metagenomic analyses enable scientists to analyze genes and hereditary series from uncultured bacteria discovered in the environment.
Using the genes and hereditary series offered by Banfield, Banda, and Shih effectively revealed type I’ rubisco in the laboratory utilizing E. coli. To discover how this freshly determined type functions and how it compares to formerly found rubisco enzymes, the researchers required to construct accurate, 3D designs of its structure. For this job, the lead authors relied on Berkeley Lab structural biologists Paul Adams, Henrique Pereira, and Michal Hammel.
First, Adams and Pereira carried out X-ray crystallography – a method that can produce pictures of particles with atomic-level resolution – at Berkeley Lab’s Advanced Light Source (ALS). Then, to catch how the enzyme’s structure modifications throughout various states of activity, Hammel used a strategy called small-angle X-ray scattering (SAXS) utilizing the SIBYLS beamline at the ALS.
SAXS is a lower-resolution strategy, however unlike crystallography – which needs that sample particles are frozen in crystal type – SAXS is carried out in option. When the information from the 2 methods are integrated, researchers can build extraordinary designs of complex particles as they appear in nature.
“Like many enzymes key to life, rubisco has several protein domains connected together, and as it binds with other molecules during the photosynthesis reaction, it will cycle through different arrangements of those domains,” stated Hammel, a biophysicist in Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging (MBIB) Division. “Our techniques really worked hand-in-hand to reveal how this new, novel rubisco behaves in real-world, physiological conditions.”
The ALS examinations revealed that like type I rubisco, form I’ rubisco is developed from 8 big subunits. However, it doesn’t have the little subunits that were formerly believed to be important to its carbon-fixing function.
The scientists now think that type I’ rubisco represents a missing out on link in the evolutionary history of type I rubisco’s structure.
“The discovery of an octameric rubisco that forms without little subunits enables us to ask [evolutionary] concerns about what life would’ve appeared like without the performance imparted by little subunits,” stated Banda.
Following the success of the structural examination into type I’ rubisco, Shih has actually employed Hammel, Adams, and Pereira to use their complementary technique for research studies of other essential plant enzymes, consisting of extra types of rubisco.
“We’ve been working together at Berkeley Lab for over 10 years now, and it was really satisfying to be able to see what crystallography and SAXS combined can do to understand biology problems,” stated Pereira, an MBIB biophysicist. “Once, the scientists who use these different structural biology techniques would have seen themselves as in competition, racing each other to solve structures. But now it’s pure collaboration.”
Read Missing Link Discovered in the Evolution of Photosynthesis and Carbon Fixation for more on this research study.
Reference: “Novel bacterial clade reveals origin of form I Rubisco” by Douglas M. Banda, Jose H. Pereira, Albert K. Liu, Douglas J. Orr, Michal Hammel, Christine He, Martin A. J. Parry, Elizabete Carmo-Silva, Paul D. Adams, Jillian F. Banfield and Patrick M. Shih, 31 August 2020, Nature Plants.
DOI: 10.1038/s41477-020-00762-4
The ALS is a Department of Energy (DOE) user center and JBEI is a DOE Bioenergy Research Center. The crystallography beamline utilized in this research study is run by the Berkeley Center for Structural Biology and moneyed by the Howard Hughes Medical Institute. The SIBYLS beamline is supported by the National Cancer Institute grant Structural Biology of DNA Repair and the DOE Office of Science. This work was supported in part by the DOE Office of Science.