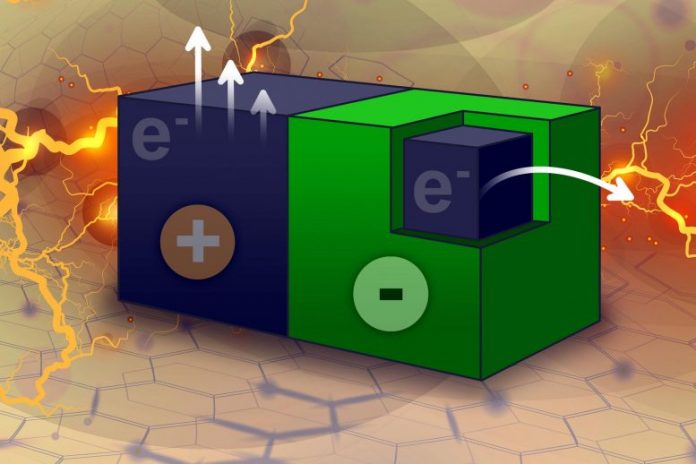
MIT engineers have actually found a method to create electrical power utilizing small carbon particles that can produce an electrical existing merely by connecting with a natural solvent in which they’re drifting. The particles are made from crushed carbon nanotubes (blue) covered with a Teflon-like polymer (green). Credit: Jose-Luis Olivares, MIT. Based on a figure thanks to the scientists.
Tiny Particles Power Chemical Reactions
A brand-new product made from carbon nanotubes can create electrical power by scavenging energy from its environment.
MIT engineers have actually found a brand-new method of creating electrical power utilizing small carbon particles that can produce an existing merely by connecting with liquid surrounding them.
The liquid, a natural solvent, draws electrons out of the particles, creating an existing that might be utilized to drive chain reactions or to power micro- or nanoscale robotics, the scientists state.
“This mechanism is new, and this way of generating energy is completely new,” states Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT. “This technology is intriguing because all you have to do is flow a solvent through a bed of these particles. This allows you to do electrochemistry, but with no wires.”
In a brand-new research study explaining this phenomenon, the scientists revealed that they might utilize this electrical existing to drive a response called alcohol oxidation — a natural chain reaction that is necessary in the chemical market.
Strano is the senior author of the paper, which appears today (June 7, 2021) in Nature Communications. The lead authors of the research study are MIT college student Albert Tianxiang Liu and previous MIT scientist Yuichiro Kunai. Other authors consist of previous college student Anton Cottrill, postdocs Amir Kaplan and Hyunah Kim, college student Ge Zhang, and current MIT graduates Rafid Mollah and Yannick Eatmon.
Unique residential or commercial properties
The brand-new discovery outgrew Strano’s research study on carbon nanotubes — hollow tubes made from a lattice of carbon atoms, which have distinct electrical residential or commercial properties. In 2010, Strano showed, for the very first time, that carbon nanotubes can create “thermopower waves.” When a carbon nanotube is covered with layer of fuel, moving pulses of heat, or thermopower waves, travel along television, producing an electrical existing.
That work led Strano and his trainees to discover an associated function of carbon nanotubes. They discovered that when part of a nanotube is covered with a Teflon-like polymer, it develops an asymmetry that makes it possible for electrons to stream from the covered to the uncoated part of television, creating an electrical existing. Those electrons can be extracted by immersing the particles in a solvent that is starving for electrons.
To harness this unique ability, the scientists produced electricity-generating particles by grinding up carbon nanotubes and forming them into a sheet of paper-like product. One side of each sheet was covered with a Teflon-like polymer, and the scientists then eliminated little particles, which can be any shape or size. For this research study, they made particles that were 250 microns by 250 microns.
When these particles are immersed in a natural solvent such as acetonitrile, the solvent stick to the uncoated surface area of the particles and starts pulling electrons out of them.
“The solvent takes electrons away, and the system tries to equilibrate by moving electrons,” Strano states. “There’s no sophisticated battery chemistry inside. It’s just a particle and you put it into solvent and it starts generating an electric field.”
“This research cleverly shows how to extract the ubiquitous (and often unnoticed) electric energy stored in an electronic material for on-site electrochemical synthesis,” states Jun Yao, an assistant teacher of electrical and computer system engineering at the University of Massachusetts at Amherst, who was not associated with the research study. “The beauty is that it points to a generic methodology that can be readily expanded to the use of different materials and applications in different synthetic systems.”
Particle power
The existing variation of the particles can create about 0.7 volts of electrical power per particle. In this research study, the scientists likewise revealed that they can form selections of numerous particles in a little test tube. This “packed bed” reactor creates enough energy to power a chain reaction called an alcohol oxidation, in which an alcohol is transformed to an aldehyde or a ketone. Usually, this response is not carried out utilizing electrochemistry due to the fact that it would need excessive external existing.
“Because the packed bed reactor is compact, it has more flexibility in terms of applications than a large electrochemical reactor,” Zhang states. “The particles can be made very small, and they don’t require any external wires in order to drive the electrochemical reaction.”
In future work, Strano wants to utilize this type of energy generation to develop polymers utilizing just co2 as a beginning product. In an associated task, he has actually currently produced polymers that can regrow themselves utilizing co2 as a structure product, in a procedure powered by solar power. This work is motivated by carbon fixation, the set of chain reactions that plants utilize to develop sugars from co2, utilizing energy from the sun.
In the longer term, this method might likewise be utilized to power micro- or nanoscale robotics. Strano’s laboratory has actually currently started developing robotics at that scale, which might one day be utilized as diagnostic or ecological sensing units. The concept of having the ability to scavenge energy from the environment to power these sort of robotics is appealing, he states.
“It means you don’t have to put the energy storage on board,” he states. “What we like about this mechanism is that you can take the energy, at least in part, from the environment.”
Reference: “Solvent-induced electrochemistry at an electrically asymmetric carbon Janus particle” by Albert Tianxiang Liu, Yuichiro Kunai, Anton L. Cottrill, Amir Kaplan, Ge Zhang, Hyunah Kim, Rafid S. Mollah, Yannick L. Eatmon and Michael S. Strano, 7 June 2021, Nature Communications.
DOI: 10.1038/s41467-021-23038-7
The research study was moneyed by the U.S. Department of Energy and a seed grant from the MIT Energy Initiative.