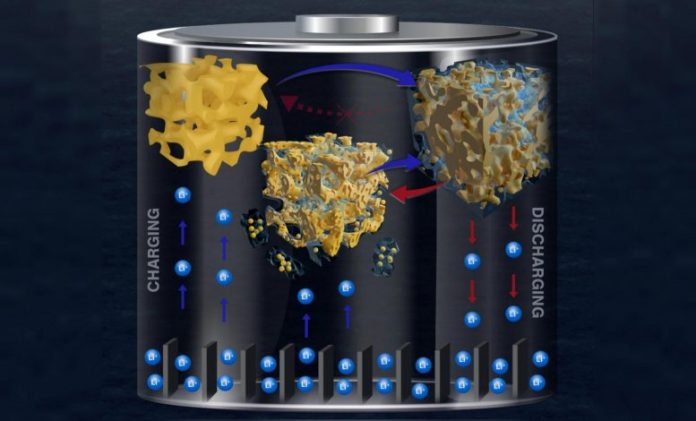
A current research study from Penn Engineering scientists offers brand-new insights into why silicon anodes in lithium-ion batteries quickly break down and stop working. Using advanced imaging strategies and high-contrast gold to stand in for silicon, they’ve demonstrated how pieces of the anode are caught in the chemical layer that forms in each cycle, slowly burrowing the anode till it breaks down. Credit: Penn Engineering
Rechargeable lithium-ion batteries are common, powering smart devices, tablets, laptop computers and, progressively, electrical cars. Making these batteries lighter, smaller sized, less expensive and able to charge much faster, all without compromising efficiency, is for that reason a significant style obstacle. To tackle this issue, researchers and engineers are establishing brand-new electrode products that can save higher quantities of lithium in the very same quantity of area.
One appealing option is making use of alloy-type products in a battery’s unfavorable electrode, likewise referred to as the anode. For example, one pound of silicon — which produces an “alloy-type” anode — can save about the very same quantity of lithium as 10 pounds of graphite, which is discovered in the “intercalation-type” anodes presently utilized in industrial lithium-ion batteries. This implies that changing the latter with the previous might possibly make the anode 10 times lighter and significantly smaller sized.
Despite this guarantee, alloy-type anodes have actually not seen extensive adoption. This is partially since when lithium ions are placed into the alloy-type silicon particles within the anode, those particles start to broaden and disintegrate, leading to a battery that stops working after just a few charging cycles. Reducing the size of these particles so their functions are at the nanoscale — such as in nanoporous silicon — reduces this type of deterioration, however the real systems at play are not completely comprehended.
Now, in a research study released in AIR CONDITIONING Energy Letters, Penn Engineering scientists have actually exposed the complex electrochemical procedure that happens at the nanoscale when alloy-type anodes charge and discharge. A much better understanding of the deterioration habits that is presently hampering this appealing class of energy storage products might unlock to brand-new, more effective battery styles.
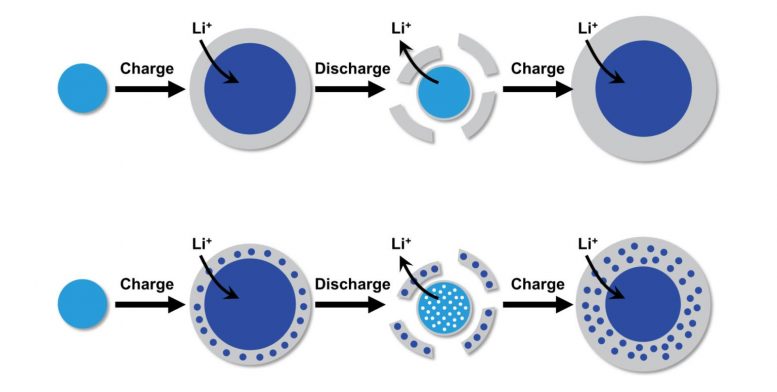
The standard design for how basic alloy-type anode deterioration happens prior to this research study is displayed in the leading part of this illustration. When a lithium-ion battery with a silicon anode charges, particles of silicon (light blue) physically grow as they take in lithium ions. A layer of SEI, or solid-electrolyte interphase (gray), likewise forms around these lithium-containing silicon particles (dark blue), just to break off when the battery discharges. This research study offers brand-new insights into the reason for the deterioration, as seen in the bottom part of the illustration. During charging, pieces of silicon end up being caught in the SEI, leaving the initial particle permeable when the SEI separates from it throughout discharging. As this procedure repeats, the particle diminishes increasingly more, till it eventually breaks down. Credit: Penn Engineering
The research study was carried out by Eric Detsi, Stephenson Term Assistant Professor in the department of Materials Science and Engineering (MSE), together with graduate research study assistants John Corsi and Samuel Welborn. They teamed up with Eric Stach, teacher in MSE and director of the Laboratory for Research on the Structure of Matter (LRSM).
As their name recommends, lithium-ion batteries save energy through an electrochemical response in between lithium from the favorable electrode, likewise referred to as the cathode, and the product in their anode. As lithium ions physically get in the areas in the anode’s lattice throughout charging, they bond with that product and soak up electrons at the same time; releasing the battery eliminates the lithium so the procedure can be duplicated, however when it comes to alloy-type anodes, likewise triggers the anode product to grow and ultimately disintegrate.
There are numerous intermediary actions in these procedures; comprehending how they vary in between thick silicon and nanoporous silicon may provide some tip regarding why the latter much better withstands deterioration. However, close examination of these procedures in action has actually been stymied by obstacles in imaging the appropriate silicon structures at such little scales.
“To address this challenge,” Detsi states, “we used a unique combination of transmission electron microscopy and X-ray scattering techniques to study the degradation of lithium-ion battery anodes during charging and discharging.”
“We used gold instead of silicon because gold yields better contrast during electron microscopy imaging than silicon,” includes Welborn, “which allows for clear detection of the solid-electrolyte interphase surface coating, known as SEI, that forms on the gold electrode during charging and discharging. Gold also scatters more X-rays than silicon, which makes it easier to probe changes to the anode structure during those processes.”
For this research study, the group utilized the electron microscopy center at the Singh Center for Nanotechnology, along with the Penn Dual Source and Environmental X-ray Scattering (DEXS) center in the LRSM. The arises from these 2 strategies formed an abundant dataset that permitted the scientists to upgrade the formerly comprehended design for how this deterioration procedure happens.
These instruments permitted the group to recognize the vital action throughout discharge: the development of a thick SEI layer on the permeable gold surface area.
“As lithium is stored in gold, the volume of the metallic gold ligaments in the nanoporous structure rapidly expands, eventually breaking,” Corsi states. “These fractured ligament pieces become trapped within the surrounding SEI layer. When the process is reversed, the ligaments contract as lithium is removed and this volume change causes the SEI layer containing trapped material to crack and separate from the rest of the electrode.”
As the battery is charged once again, a fresh SEI layer grows on the surface area, gathering more fractured pieces of the electrode. This damage collects over duplicated charging cycles, with big pieces of the electrode ultimately splitting off and triggering the battery to quickly stop working.
The scientists think that the insights gotten for nanoporous gold have extensive ramifications for other extremely studied, guaranteeing alloy-type anode products such as silicon and tin. Understanding the systems for how these anodes break down gradually will enable scientists to create lasting, high-energy-density battery products.
Reference: “Insights into the Degradation Mechanism of Nanoporous Alloy-Type Li-Ion Battery Anodes” by John S. Corsi, Samuel S. Welborn, Eric A. Stach and Eric Detsi, 12 April 2021, AIR CONDITIONING Energy Letters.
DOI: 10.1021/acsenergylett.1c00324
This research study was supported by the National Science Foundation (NSF) (DMR-1720530) and Vagelos Institute for Energy Science and Technology (VIEST) through a 2018 VIEST graduate fellowship.