Single-cell analysis of autopsy samples from COVID-19 clients demonstrates how the lungs consistently attempted, and stopped working, to fix themselves.
Scientists from numerous healthcare facilities and proving ground have actually revealed what occurs in specific cells of clients who passed away of COVID-19. In a research study released in Nature, the scientists explain how contaminated cells from several organs showed a series of molecular and genomic modifications. They likewise saw indications of several, not successful efforts by the lungs to fix themselves in reaction to breathing failure, which is the leading cause of death in COVID-19 clients.
“You really feel the tragedy of the disease when you see that result,” stated Aviv Regev, co-senior author of the research study and a core institute member at the Broad Institute of MIT and Harvard when the research study started. “The lung tries everything at its disposal, and it still can’t fix itself. This was a very emotional study. We are grateful to the patients and families who agreed to donate tissue for COVID-19 research to help advance understanding of this devastating disease.”
The scientists studied tissue acquired at autopsies of 17 people who caught COVID-19 and were taken care of at Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital.
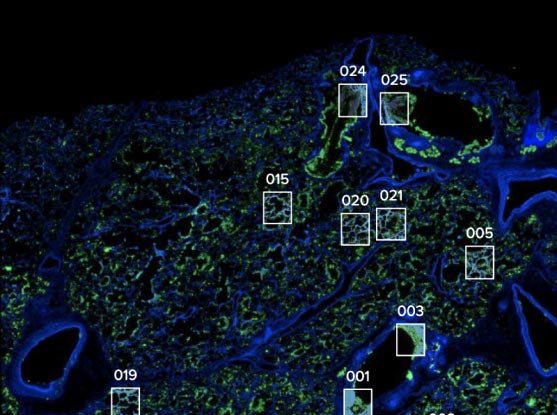
Researchers profiled lung tissue from departed COVID-19 clients and focused on crucial areas and structures of interest. Credit: Domenic Abbondanza
The group examined how the SARS-CoV-2 infection hinders the function of cells and their hereditary programs. They utilized single-cell RNA sequencing information from tissue samples drawn from 11 organ systems—consisting of the lungs, heart, liver, and kidneys—to construct a detailed “cell atlas” of numerous countless specific cells demonstrating how COVID-19 can result in organ failure and death.
“We knew people were passing away from COVID-related pneumonia and extrapulmonary complications,” stated Alexandra-Chloé Villani, an associate member of the Broad, a primary private investigator at Mass General, an assistant teacher of medication at Harvard Medical School, and co-senior author on the research study. “Before this study, we had limited knowledge of the cellular and molecular mechanisms that were involved in driving a patient’s demise.”
The research study information the outcomes of a cooperation of scientists from the Broad Institute, Mass General, the Ragon Institute of MGH, MIT and Harvard, MIT, Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, Columbia University Irving Medical Center, and other organizations. A group led by the Columbia partners co-authored a buddy research study that is likewise released in Nature.
The group’s cell atlas is easily and freely readily available for other researchers to check out. They likewise developed a 420-specimen biobank from the autopsy samples that can be utilized for other COVID-19 research studies. “We created a foundational resource for other researchers to use in the future to ask specific questions,” stated Orit Rozenblatt-Rosen, co-senior author and an institute researcher and the clinical director of the Klarman Cell Observatory at the Broad when the research study started. “Hopefully our findings will allow people to find better therapeutics for COVID-19.”
Novel Techniques for a Novel Virus
To discover cellular systems underlying organ failure brought on by COVID-19, the scientists understood they required to study the organs themselves. For that, they would require samples from autopsies.
Working with autopsy samples is challenging under typical situations. To handle samples that may bring an unique, extremely infectious pathogen, the scientists established brand-new tissue collection and processing procedures suitable with requirements for a Biosafety Level 3 laboratory.
“We wanted to ensure we could learn and share as much as humanly possible to help prevent future deaths, while prioritizing the safety and well-being of all involved. This was no small feat, given COVID-related restrictions and all the surrounding uncertainties. It was amazing to see dozens of scientists and medical professionals from several institutes come together as a collaborative partnership to carefully design and coordinate our experimental and computational efforts,” stated institute member and co-senior author Alex K. Shalek, who is likewise a member of the Ragon Institute, and an associate teacher of chemistry, a core member of the Institute for Medical Engineering and Science, and an extramural member of the Koch Institute for Integrative Cancer Research at MIT.
The group then profiled RNA from the specific cells and established brand-new approaches to evaluate and annotate the big quantities of series information. They compared gene expression signatures from various cells: COVID-19-harmed cells and uninfected cells from the COVID-19 clients, in addition to cells from clients with other illness and from healthy people.
Havoc in the Lungs
The most substantial suite of findings were from the lungs. The researchers were surprised by the level of the modifications in hereditary programs they discovered there. “The virus wreaks havoc in the lungs and we see it in the cells,” Regev stated.
One primary reason for lung damage in COVID-19 is the damage of AT1 cells, which make it possible for breathing and gas transfer. The researchers discovered that as AT1 cells passed away, associated lung cells called AT2 tried to transform themselves into AT1 cells through a procedure called transdifferentiation. But this effort stopped mid-way through, leaving the cells in an intermediary state that is typically seen in clients with other lung illness such as lung fibrosis.
In a desperate effort at self-repair, the lungs attempted to turn cells from greater up in the air passages, called intrapulmonary basal-like progenitor cells, into AT1 cells. This effort at transdifferentiation had actually just formerly been seen in mouse designs.
The findings recommend that the lung failure in clients was brought on by the failure of lung cells to outmatch the damage brought on by the infection as the cells attempted to regrow.
Changing Programs
The paper likewise explains how the infection effects other tissues beyond the lungs. One unexpected finding was that while the heart sustained considerable damage and revealed proof of modified hereditary programs in various cell types, there was extremely little viral RNA in the heart tissue itself. “Whether that means the virus had already been cleared, or that the heart was collateral damage is an area for further research,” stated Regev.
The scientists likewise took a look at 27 various genes that previous genome-wide association research studies have actually connected to extreme COVID-19. They zeroed in on a handful that were extremely revealed in crucial cell enters the brand-new research study, especially those in contaminated lungs. This finding assists limit the list of prospective hereditary aspects for extreme illness and highlights the cell types that might be most pertinent in extreme COVID-19.
The group now prepares to complete evaluating the other autopsied tissues, such as brain, spleen and trachea, to paint a more total image of COVID-19 pathology and supply a resource for future research studies.
For more on this research study, read New Cell Atlas of COVID Lungs Reveals Why SARS-CoV-2 Is Different and Deadly.
Reference: “COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets” by Toni M. Delorey, Carly G. K. Ziegler, Graham Heimberg, Rachelly Normand, Yiming Yang, Åsa Segerstolpe, Domenic Abbondanza, Stephen J. Fleming, Ayshwarya Subramanian, Daniel T. Montoro, Karthik A. Jagadeesh, Kushal K. Dey, Pritha Sen, Michal Slyper, Yered H. Pita-Juárez, Devan Phillips, Jana Biermann, Zohar Bloom-Ackermann, Nikolaos Barkas, Andrea Ganna, James Gomez, Johannes C. Melms, Igor Katsyv, Erica Normandin, Pourya Naderi, Yury V. Popov, Siddharth S. Raju, Sebastian Niezen, Linus T.-Y. Tsai, Katherine J. Siddle, Malika Sud, Victoria M. Tran, Shamsudheen K. Vellarikkal, Yiping Wang, Liat Amir-Zilberstein, Deepak S. Atri, Joseph Beechem, Olga R. Brook, Jonathan Chen, Prajan Divakar, Phylicia Dorceus, Jesse M. Engreitz, Adam Essene, Donna M. Fitzgerald, Robin Fropf, Steven Gazal, Joshua Gould, John Grzyb, Tyler Harvey, Jonathan Hecht, Tyler Hether, Judit Jané-Valbuena, Michael Leney-Greene, Hui Ma, Cristin McCabe, Daniel E. McLoughlin, Eric M. Miller, Christoph Muus, Mari Niemi, Robert Padera, Liuliu Pan, Deepti Pant, Carmel Pe’er, Jenna Pfiffner-Borges, Christopher J. Pinto, Jacob Plaisted, Jason Reeves, Marty Ross, Melissa Rudy, Erroll H. Rueckert, Michelle Siciliano, Alexander Sturm, Ellen Todres, Avinash Waghray, Sarah Warren, Shuting Zhang, Daniel R. Zollinger, Lisa Cosimi, Rajat M. Gupta, Nir Hacohen, Hanina Hibshoosh, Winston Hide, Alkes L. Price, Jayaraj Rajagopal, Purushothama Rao Tata, Stefan Riedel, Gyongyi Szabo, Timothy L. Tickle, Patrick T. Ellinor, Deborah Hung, Pardis C. Sabeti, Richard Novak, Robert Rogers, Donald E. Ingber, Z. Gordon Jiang, Dejan Juric, Mehrtash Babadi, Samouil L. Farhi, Benjamin Izar, James R. Stone, Ioannis S. Vlachos, Isaac H. Solomon, Orr Ashenberg, Caroline B. M. Porter, Bo Li, Alex K. Shalek, Alexandra-Chloé Villani, Orit Rozenblatt-Rosen and Aviv Regev, 29 April 2021, Nature.
DOI: 10.1038/s41586-021-03570-8
Aviv Regev is now Executive Vice President, Genentech Research and Early Development.
Orit Rozenblatt-Rosen is now Executive Director and Head of Cellular and Tissue Genomics at Genentech.
Support for this research study was offered in part by the Manton Foundation, Klarman Family Foundation, Howard Hughes Medical Institute, the Chan Zuckerberg Initiative, and the Human Tumor Atlas Network trans-network tasks SARDANA (Shared Repositories, Data, Analysis and Access), DARPA, and the United States Food and Drug Administration.





