By
Use of an unique electrolyte might enable sophisticated metal electrodes and greater voltages, increasing capability and cycle life.
Lithium-ion batteries have actually enabled the light-weight electronic gadgets whose mobility we now consider given, in addition to the quick growth of electrical car production. But scientists around the globe are continuing to press limitations to attain ever-greater energy densities — the quantity of energy that can be kept in an offered mass of product — in order to enhance the efficiency of existing gadgets and possibly allow brand-new applications such as long-range drones and robotics.
One appealing method is using metal electrodes in location of the standard graphite, with a greater charging voltage in the cathode. Those efforts have actually been obstructed, nevertheless, by a range of undesirable chain reaction that accompany the electrolyte that separates the electrodes. Now, a group of scientists at MIT and in other places has actually discovered an unique electrolyte that gets rid of these issues and might allow a substantial leap in the power-per-weight of next-generation batteries, without compromising the cycle life.
The research study is reported in the journal Nature Energy in a paper by MIT teachers Ju Li, Yang Shao-Horn, and Jeremiah Johnson; postdoc Weijiang Xue; and 19 others at MIT, 2 nationwide labs, and in other places. The scientists state the finding might make it possible for lithium-ion batteries, which now normally can keep about 260 watt-hours per kg, to keep about 420 watt-hours per kg. That would equate into longer varieties for electrical automobiles and longer-lasting modifications on portable gadgets.
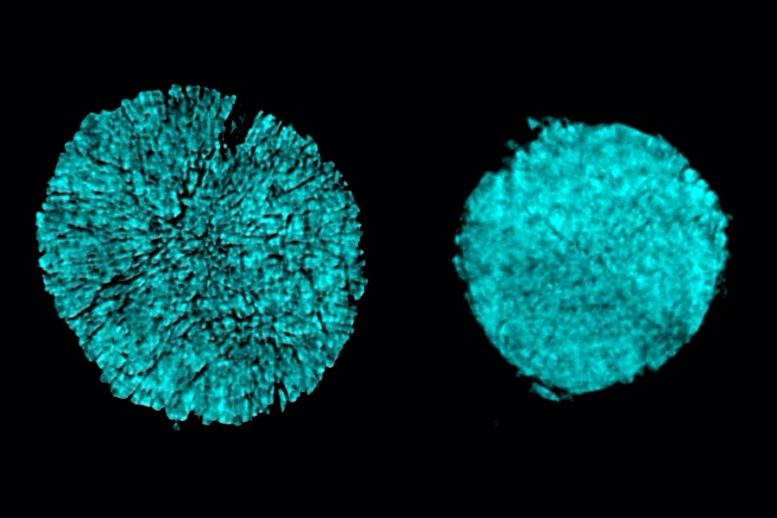
X-ray tomography images taken at Brookhaven National Lab reveal breaking of a particle in one electrode of a battery cell that utilized a standard electrolyte (as seen left wing). The scientists discovered that utilizing an unique electrolyte avoided the majority of this breaking (right). Credit: Courtesy of the scientists
The standard basic materials for this electrolyte are low-cost (though among the intermediate substances is still expensive due to the fact that it’s in minimal usage), and the procedure to make it is easy. So, this advance might be carried out reasonably rapidly, the scientists state.
The electrolyte itself is not brand-new, discusses Johnson, a teacher of chemistry. It was established a couple of years earlier by some members of this research study group, however for a various application. It became part of an effort to establish lithium-air batteries, which are viewed as the supreme long-lasting service for taking full advantage of battery energy density. But there are lots of barriers still dealing with the advancement of such batteries, which innovation might still be years away. In the meantime, using that electrolyte to lithium-ion batteries with metal electrodes ends up being something that can be attained a lot more rapidly.
The brand-new application of this electrode product was discovered “somewhat serendipitously,” after it had actually at first been established a couple of years earlier by Shao-Horn, Johnson, and others, in a collective endeavor targeted at lithium-air battery advancement.
“There’s still really nothing that allows a good rechargeable lithium-air battery,” Johnson states. However, “we designed these organic molecules that we hoped might confer stability, compared to the existing liquid electrolytes that are used.” They established 3 various sulfonamide-based solutions, which they discovered were rather resistant to oxidation and other destruction results. Then, dealing with Li’s group, postdoc Xue chose to attempt this product with more basic cathodes rather.
The kind of battery electrode they have actually now utilized with this electrolyte, a nickel oxide including some cobalt and manganese, “is the workhorse of today’s electric vehicle industry,” states Li, who is a teacher of nuclear science and engineering and products science and engineering.
Because the electrode product broadens and contracts anisotropically as it gets charged and released, this can result in breaking and a breakdown in efficiency when utilized with standard electrolytes. But in experiments in cooperation with Brookhaven National Laboratory, the scientists discovered that utilizing the brand-new electrolyte dramatically minimized these stress-corrosion breaking deteriorations.
The issue was that the metal atoms in the alloy tended to liquify into the liquid electrolyte, losing mass and causing breaking of the metal. By contrast, the brand-new electrolyte is very resistant to such dissolution. Looking at the information from the Brookhaven tests, Li states, it was “sort of shocking to see that, if you just change the electrolyte, then all these cracks are gone.” They discovered that the morphology of the electrolyte product is a lot more robust, and the shift metals “just don’t have as much solubility” in these brand-new electrolytes.
That was an unexpected mix, he states, due to the fact that the product still easily permits lithium ions to go through — the vital system by which batteries get charged and released — while obstructing the other cations, referred to as shift metals, from getting in. The build-up of undesirable substances on the electrode surface area after lots of charging-discharging cycles was minimized more than significantly compared to the basic electrolyte.
“The electrolyte is chemically resistant against oxidation of high-energy nickel-rich materials, preventing particle fracture and stabilizing the positive electrode during cycling,” states Shao-Horn, a teacher of mechanical engineering and products science and engineering. “The electrolyte also enables stable and reversible stripping and plating of lithium metal, an important step toward enabling rechargeable lithium-metal batteries with energy two times that of the state-the-art lithium-ion batteries. This finding will catalyze further electrolyte search and designs of liquid electrolytes for lithium-metal batteries rivaling those with solid state electrolytes.”
The next action is to scale the production to make it inexpensive. “We make it in one very easy reaction from readily available commercial starting materials,” Johnson states. Right now, the precursor substance utilized to manufacture the electrolyte is pricey, however he states, “I think if we can show the world that this is a great electrolyte for consumer electronics, the motivation to further scale up will help to drive the price down.”
Because this is basically a “drop in” replacement for an existing electrolyte and doesn’t need redesign of the whole battery system, Li states, it might be carried out rapidly and might be advertised within a number of years. “There’s no expensive elements, it’s just carbon and fluorine. So it’s not limited by resources, it’s just the process,” he states.
Reference: “Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte” by Weijiang Xue, Mingjun Huang, Yutao Li, Yun Guang Zhu, Rui Gao, Xianghui Xiao, Wenxu Zhang, Sipei Li, Guiyin Xu, Yang Yu, Peng Li, Jeffrey Lopez, Daiwei Yu, Yanhao Dong, Weiwei Fan, Zhe Shi, Rui Xiong, Cheng-Jun Sun, Inhui Hwang, Wah-Keat Lee, Yang Shao-Horn, Jeremiah A. Johnson and Ju Li, Nature Energy.
DOI: 10.1038/s41560-021-00792-y
The research study was supported by the U.S. Department of Energy and the National Science Foundation, and utilized centers at Brookhaven National Laboratory and Argonne National Laboratory.





