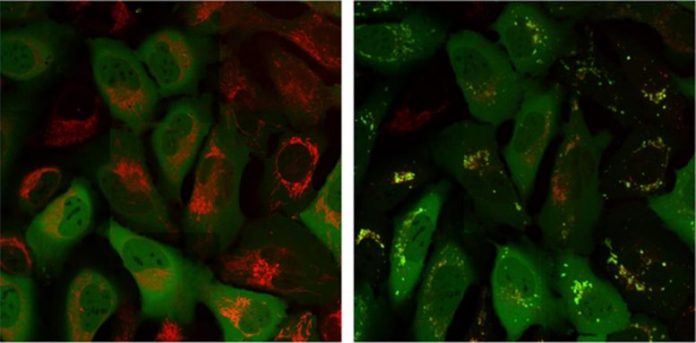
Parkin protein (green signal) remains in a various part of the cell than the mitochondria (red signal) sometimes 0 (left image) however then co-localizes with the mitochondria after 60 minutes (ideal image). Credit: Salk Institute
Enzyme with main function in cancer and type 2 diabetes likewise triggers “clean-up” protein in Parkinson’s.
When cells are stressed out, chemical alarms go off, setting in movement a flurry of activity that secures the cell’s essential gamers. During the rush, a protein called Parkin rushes to secure the mitochondria, the power stations that produce energy for the cell. Now Salk scientists have actually found a direct link in between a master sensing unit of cell tension and Parkin itself. The very same path is likewise connected to type 2 diabetes and cancer, which might open a brand-new opportunity for dealing with all 3 illness.
“Our findings represent the earliest step in Parkin’s alarm response that anyone’s ever found by a long shot. All the other known biochemical events happen at one hour; we’ve now found something that happens within five minutes,” states Professor Reuben Shaw, director of the NCI-designated Salk Cancer Center and senior author of the brand-new work, detailed in Science Advances on April 7, 2021. “Decoding this major step in the way cells dispose of defective mitochondria has implications for a number of diseases.”
Parkin’s task is to remove mitochondria that have actually been harmed by cellular tension so that brand-new ones can take their location, a procedure called mitophagy. However, Parkin is altered in familial Parkinson’s illness, making the protein not able to remove harmed mitochondria. While researchers have actually understood for a long time that Parkin in some way senses mitochondrial tension and starts the procedure of mitophagy, nobody comprehended precisely how Parkin was very first noticing issues with the mitochondria—Parkin in some way understood to move to the mitochondria after mitochondrial damage, however there was no recognized signal to Parkin till after it showed up there.
Shaw’s laboratory, which is popular for their operate in the fields of metabolic process and cancer, invested years extremely looking into how the cell controls a more basic procedure of cellular cleansing and recycling called autophagy. About 10 years back, they found that an enzyme called AMPK, which is extremely conscious cellular tension of lots of kinds, consisting of mitochondrial damage, manages autophagy by triggering an enzyme called ULK1.
Following that discovery, Shaw and college student Portia Lombardo started looking for autophagy-related proteins straight triggered by ULK1. They evaluated about 50 various proteins, anticipating about 10 percent to fit. They were surprised when Parkin topped the list. Biochemical paths are typically extremely complicated, including approximately 50 individuals, each triggering the next. Finding that a procedure as essential as mitophagy is started by just 3 individuals—very first AMPK, then ULK1, then Parkin—was so unexpected that Shaw might hardly think it.
To validate the findings were proper, the group utilized mass spectrometry to expose specifically where ULK1 was connecting a phosphate group to Parkin. They discovered that it landed in a brand-new area other scientists had actually just recently discovered to be vital for Parkin activation however hadn’t understood why. A postdoctoral fellow in Shaw’s laboratory, Chien-Min Hung, then did exact biochemical research studies to show each element of the timeline and defined which proteins were doing what, and where. Shaw’s research study now starts to discuss this essential initial step in Parkin activation, which Shaw assumes might work as a “heads-up” signal from AMPK down the hierarchy through ULK1 to Parkin to go have a look at the mitochondria after a very first wave of inbound damage, and, if required, activate damage of those mitochondria that are too seriously harmed to gain back function.
The findings have extensive ramifications. AMPK, the main sensing unit of the cell’s metabolic process, is itself triggered by a growth suppressor protein called LKB1 that is associated with a variety of cancers, as developed by Shaw in previous work, and it is triggered by a type 2 diabetes drug called metformin. Meanwhile, various research studies reveal that diabetes clients taking metformin display lower threats of both cancer and aging comorbidities. Indeed, metformin is presently being pursued as one of the very first “anti-aging” rehabs in scientific trials.
“The big takeaway for me is that metabolism and changes in the health of your mitochondria are critical in cancer, they’re critical in diabetes, and they’re critical in neurodegenerative diseases,” states Shaw, who holds the William R. Brody Chair. “Our finding says that a diabetes drug that activates AMPK, which we previously showed can suppress cancer, may also help restore function in patients with neurodegenerative disease. That’s because the general mechanisms that underpin the health of the cells in our bodies are way more integrated than anyone could have ever imagined.”
Reference: “AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy” by Chien-Min Hung, Portia S. Lombardo, Nazma Malik, Sonja N. Brun, Kristina Hellberg, Jeanine L. Van Nostrand, Daniel Garcia, Joshua Baumgart, Ken Diffenderfer, John M. Asara and Reuben J. Shaw, 7 April 2021, Science Advances.
DOI: 10.1126/sciadv.abg4544