Quadruple-helix DNA structure. Credit: Imperial College London
New probes permit researchers to see four-stranded DNA engaging with particles inside living human cells, deciphering its function in cellular procedures.
DNA typically forms the timeless double helix shape of 2 hairs wound around each other. While DNA can form some more unique shapes in test tubes, couple of are seen in genuine living cells.
“G-quadruplexes play an important role in a wide variety of processes vital for life, and in a range of diseases, but the missing link has been imaging this structure directly in living cells.” — Ben Lewis
However, four-stranded DNA, called G-quadruplex, has actually just recently been seen forming naturally in human cells. Now, in brand-new research study released in Nature Communications, a group led by Imperial College London researchers have actually developed brand-new probes that can see how G-quadruplexes are engaging with other particles inside living cells.
G-quadruplexes are discovered in greater concentrations in cancer cells, so are believed to contribute in the illness. The probes expose how G-quadruplexes are ‘unwound’ by specific proteins, and can likewise assist determine particles that bind to G-quadruplexes, causing possible brand-new drug targets that can interrupt their activity.
Needle in a haystack
One of the lead authors, Ben Lewis, from the Department of Chemistry at Imperial, stated: “A various DNA shape will have a massive influence on all procedures including it – such as reading, copying, or revealing hereditary details.
“Evidence has been mounting that G-quadruplexes play an important role in a wide variety of processes vital for life, and in a range of diseases, but the missing link has been imaging this structure directly in living cells.”
G-quadruplexes are unusual inside cells, suggesting basic methods for discovering such particles have trouble discovering them particularly. Ben Lewis explains the issue as “like finding a needle in a haystack, but the needle is also made of hay”.
To resolve the issue, scientists from the Vilar and Kuimova groups in the Department of Chemistry at Imperial coordinated with the Vannier group from the Medical Research Council’s London Institute of Medical Sciences.
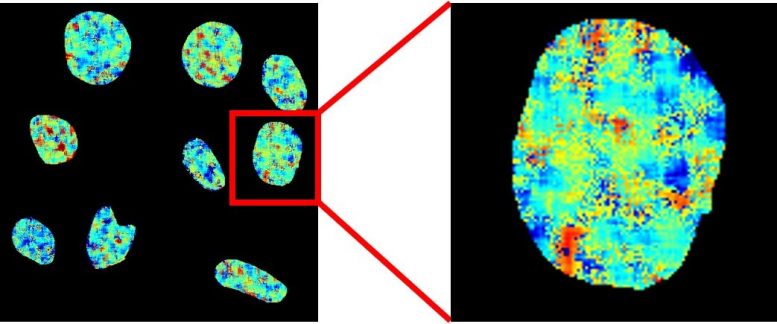
Fluorescence life time imaging microscopy map of nuclear DNA in live cells stained with the brand-new probe. Colours represent fluorescence life times in between 9 (red) and 13 (blue) nanoseconds. Credit: Imperial College London
They utilized a chemical probe called DAOTA-M2, which fluoresces (illuminate) in the existence of G-quadruplexes, however rather of keeping track of the brightness of fluorescence, they kept an eye on the length of time this fluorescence lasts. This signal does not depend upon the concentration of the probe or of G-quadruplexes, suggesting it can be utilized to unquestionably picture these unusual particles.
Dr. Marina Kuimova, from the Department of Chemistry at Imperial, stated: “By applying this more sophisticated approach we can remove the difficulties which have prevented the development of reliable probes for this DNA structure.”
Looking straight in live cells
The group utilized their probes to study the interaction of G-quadruplexes with 2 helicase proteins – particles that ‘unwind’ DNA structures. They revealed that if these helicase proteins were eliminated, more G-quadruplexes existed, revealing that the helicases contribute in relaxing and therefore breaking down G-quadruplexes.
“Many researchers have been interested in the potential of G-quadruplex binding molecules as potential drugs for diseases such as cancers. Our method will help to progress our understanding of these potential new drugs.” — Professor Ramon Vilar
Dr. Jean-Baptiste Vannier, from the MRC London Institute of Medical Sciences and the Institute of Clinical Sciences at Imperial, stated: “In the past we have had to rely on looking at indirect signs of the effect of these helicases, but now we take a look at them directly inside live cells.”
They likewise took a look at the capability of other particles to engage with G-quadruplexes in living cells. If a particle presented to a cell binds to this DNA structure, it will displace the DAOTA-M2 probe and decrease its life time, i.e. the length of time the fluorescence lasts.
This enables interactions to be studied inside the nucleus of living cells, and for more particles, such as those which are not fluorescent and can’t be seen under the microscopic lense, to be much better comprehended.
Professor Ramon Vilar, from the Department of Chemistry at Imperial, described: “Many researchers have been interested in the potential of G-quadruplex binding molecules as potential drugs for diseases such as cancers. Our method will help to progress our understanding of these potential new drugs.”
Peter Summers, another lead author from the Department of Chemistry at Imperial, stated: “This project has been a fantastic opportunity to work at the intersection of chemistry, biology and physics. It would not have been possible without the expertise and close working relationship of all three research groups.”
The 3 groups plan to continue interacting to enhance the homes of their probe and to check out brand-new biological issues and shine even more light on the functions G-quadruplexes play inside our living cells. The research study was moneyed by Imperial’s Excellence Fund for Frontier Research.
Reference: “Visualising G-quadruplex DNA dynamics in live cells by fluorescence lifetime imaging microscopy” by Peter A. Summers, Benjamin W. Lewis, Jorge Gonzalez-Garcia, Rosa M. Porreca, Aaron H. M. Lim, Paolo Cadinu, Nerea Martin-Pintado, David J. Mann, Joshua B. Edel, Jean Baptiste Vannier, Marina K. Kuimova and Ramon Vilar, 8 January 2021, Nature Communications.
DOI: 10.1038/s41467-020-20414-7