A research study group from Caltech and the UCLA Samueli School of Engineering has actually shown an appealing method to effectively transform co2 into ethylene — an essential chemical utilized to produce plastics, solvents, cosmetics and other essential items internationally.
The researchers established nanoscale copper wires with specifically shaped surface areas to catalyze a chain reaction that decreases greenhouse gas emissions while creating ethylene — an important chemical at the same time. Computational research studies of the response reveal the shaped driver prefers the production of ethylene over hydrogen or methane. A research study detailing the advance was released in Nature Catalysis.
“We are at the brink of fossil fuel exhaustion, coupled with global climate change challenges,” stated Yu Huang, the research study’s co-corresponding author, and teacher of products science and engineering at UCLA. “Developing materials that can efficiently turn greenhouse gases into value-added fuels and chemical feedstocks is a critical step to mitigate global warming while turning away from extracting increasingly limited fossil fuels. This integrated experiment and theoretical analysis presents a sustainable path towards carbon dioxide upcycling and utilization.”
Currently, ethylene has a worldwide yearly production of 158 million lots. Much of that is become polyethylene, which is utilized in plastic product packaging. Ethylene is processed from hydrocarbons, such as gas.
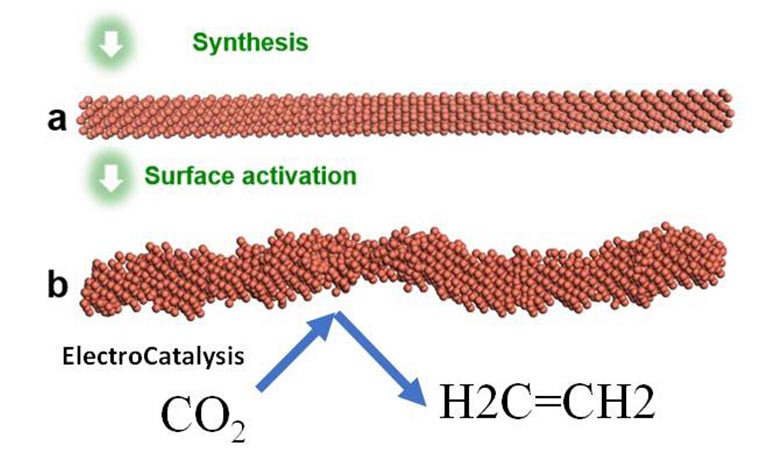
Illustration of the ElectroCatalysis system which manufactured the smooth nanowire and after that triggered it by using a voltage to get the rough stepped surface area that is extremely selective for CO2 decrease to ethylene. Credit: Yu Huang and William A. Goddard III
“The idea of using copper to catalyze this reaction has been around for a long time, but the key is to accelerate the rate so it is fast enough for industrial production,” stated William A. Goddard III, the research study’s co-corresponding author and Caltech’s Charles and Mary Ferkel Professor of Chemistry, Materials Science, and Applied Physics. “This research study reveals a strong course towards that mark, with the prospective to change ethylene production into a greener market utilizing CO2 that would otherwise wind up in the environment.”
Using copper to begin the co2 (CO2) decrease into ethylene response (C2H4) has actually suffered 2 strikes versus it. First, the preliminary chain reaction likewise produced hydrogen and methane — both unfavorable in commercial production. Second, previous efforts that led to ethylene production did not last long, with conversion effectiveness trailing off as the system continued to run.
To conquer these 2 difficulties, the scientists concentrated on the style of the copper nanowires with extremely active “steps” — comparable to a set of stairs set up at atomic scale. One interesting finding of this collective research study is that this action pattern throughout the nanowires’ surface areas stayed steady under the response conditions, contrary to basic belief that these high energy functions would ravel. This is the crucial to both the system’s resilience and selectivity in producing ethylene, rather of other final result.
The group showed a carbon dioxide-to-ethylene conversion rate of higher than 70%, far more effective than previous styles, which yielded a minimum of 10% less under the exact same conditions. The brand-new system ran for 200 hours, with little modification in conversion effectiveness, a significant advance for copper-based drivers. In addition, the thorough understanding of the structure-function relation highlighted a brand-new point of view to develop extremely active and long lasting CO2 decrease driver in action.
Huang and Goddard have actually been regular partners for several years, with Goddard’s research study group concentrating on the theoretical factors that underpin chain reactions, while Huang’s group has actually developed brand-new products and carried out experiments. The lead author on the paper is Chungseok Choi, a college student in products science and engineering at UCLA Samueli and a member of Huang’s lab.
Reference: “Highly active and steady stepped Cu surface area for boosted electrochemical CO2 decrease to C2H4” by Chungseok Choi, Soonho Kwon, Tao Cheng, Mingjie Xu, Peter Tieu, Changsoo Lee, Jin Cai, Hyuck Mo Lee, Xiaoqing Pan, Xiangfeng Duan, William A. Goddard III and Yu Huang, 7 September 2020, Nature Catalysis.
DOI: 10.1038/s41929-020-00504-x
Other authors in this research study are from UC Irvine; Soochow University, China; The Hong Kong University of Science and Technology; and the Korea Advanced Institute of Science and Technology.
The research study was supported by the Office of Naval Research, the U.S. Department of Energy, and the National Science Foundation, with extra assistance from the National Research Foundation of Korea, the Irvine Materials Research Institute and ExxonMobil.





