Sandwich with electronic spice: The illustration reveals a crystalline monoatomic gold layer under graphene (anthracite). The electronic structure of the gold layer and the graphene (green) is revealed above. The Stuttgart Max Planck scientists spectroscopically figured out the electronic residential or commercial properties by analyzing the sample with a photon beam (grey). Credit: Stiven Forti
For the very first time, it is possible to produce crystalline layers of rare-earth elements that include a single atomic layer and which are semiconducting.
Metals are normally identified by excellent electrical conductivity. This uses in specific to gold and silver. However, scientists from the Max Planck Institute for Solid State Research in Stuttgart, together with partners in Pisa and Lund, have actually now found that some rare-earth elements lose this residential or commercial property if they are thin enough. The extreme of a layer just one atom thick therefore acts like a semiconductor. This as soon as again shows that electrons act in a different way in the two-dimensional layer of a product than in three-dimensional structures. The brand-new residential or commercial properties might possibly cause applications, for instance in microelectronics and sensing unit innovation.
One may believe that gold leaf, which is just 0.1 µm thick, is really rather thin. Far from it. It can really be numerous hundred times thinner. For example, the research study group of Ulrich Starke and his previous doctoral trainee Stiven Forti have actually effectively developed a gold layer just a single atom thick. Two-dimensional gold, so to speak.
Starke is head of the Interface Analysis Facility at the Max Planck Institute for Solid State Research in Stuttgart. His group has actually long been dealing with the border in between three-dimensional (large) and two-dimensional (planar) products. Solid state scientists have an interest in this shift since it is connected with modifications in specific product residential or commercial properties. This has actually formerly been shown in two-dimensional carbon, or graphene. Among other things, its electrons are considerably more mobile and permit the electrical conductivity to increase to 30 times that of the associated three-dimensional graphite.
Gold atoms are pressed in between graphene and silicon carbide
However, for lots of metals, producing layers of product simply one atom thick is not a simple job. “With classical deposition methods, gold atoms, for example, would immediately agglomerate into three-dimensional clusters,” discusses Starke. His group is for that reason dealing with a various technique – intercalation – on which they did pioneering work around 10 years earlier. Intercalation actually suggests moving something in between. And that is specifically how it works. The scientists begin with a silicon carbide wafer. Using a procedure they established themselves, they initially transform its surface area into a single-atomic layer of graphene. “If we vaporize sublimated gold on to this silicon carbide-graphene arrangement in a high vacuum, the gold atoms migrate between the carbide and the graphene,” discusses Forti. The previous Max Planck doctoral prospect is now studying at the Center for Nanotechnology Innovation in Pisa. It is not yet completely comprehended how the thick gold atoms enter into the interstitial area. But this much is clear: greater temperature levels prefer the procedure.
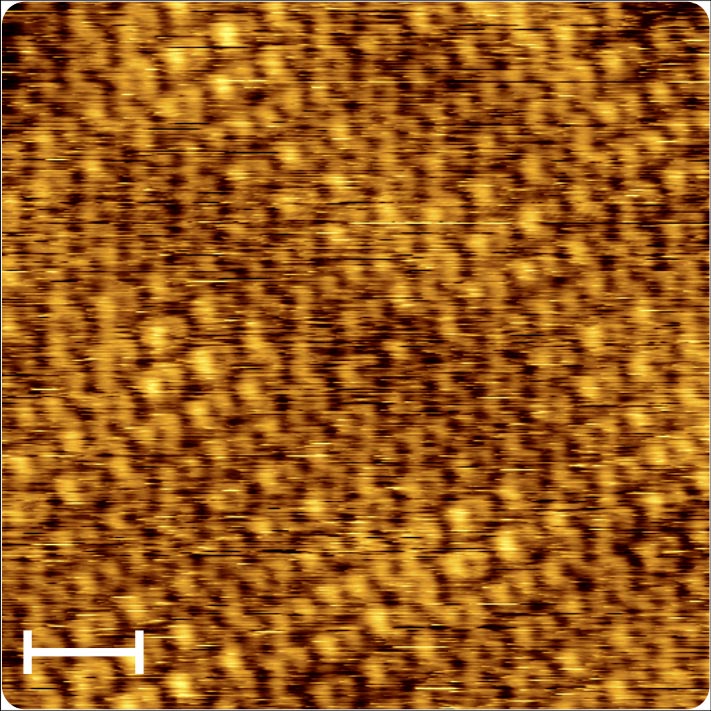
Hint at covert rare-earth element: The picture of a scanning tunnelling microscopic lense reveals graphene under which there is a crystalline gold layer a single atom thick. In addition to the hexagonal structure of the graphene, variations in brightness can be seen in the image. These occur since the gold layer engages with the graphene and forms a superlattice, the Moiré lattice. The scale bar represents one nanometer. Credit: © MPI for Solid State Research
The group had actually likewise used the intercalation method to other components, consisting of germanium, copper, and gadolinium. Yet, according to Forti, the primary focus was the impact on the residential or commercial properties of graphene. In the case of gold, nevertheless, it was discovered for the very first time that the intercalated atoms organized themselves in a routine, regularly repeating two-dimensional structure – crystalline – along the silicon carbide surface area. “If the intercalation is carried out at 600°C, the graphene layer prevents the gold atoms from agglomerating to form drops,” states Forti about the function of the carbon layer in the sandwich structure.
A gold layer including just 2 atomic layers carries out like a metal
The effective preparation of the gold layer of one atom density was just the initial step. Subsequently, the incredibly thin products and their potentially unique qualities ended up being intriguing for the scientists. They might undoubtedly reveal that the incredibly thin layer of gold establishes its own electronic – and semiconductor – residential or commercial properties. To compare: the electrical conductivity of large (i.e. three-dimensional gold) is almost as excellent as that of copper. Because theoretical factors to consider anticipate a metal character for pure 2D gold, the semiconductor finding was rather unexpected. “Interactions between the gold atoms and either the silicon carbide or the graphene carbon obviously still play a role here. This influences the energy levels of the electrons,” states Starke.
Semiconductors are necessary products in microelectronics and other fields. For example, electronic changing components such as diodes or transistors are based upon it. Starke’s group can imagine some common semiconductor applications for the brand-new 2D product. A 2nd layer of gold atoms once again offers a metal character – and therefore affects the electrical conductivity. “By varying the amount of sublimated gold, we can tightly control whether one or two layers of gold form,” discusses Forti.
It would for that reason be possible to utilize elements with rotating single- or double-atomic gold layers. The brand-new production technique would then need to be appropriately integrated with typical lithographic approaches of chip production. For example, diodes considerably smaller sized than traditional ones might be produced. According to Starke, the various electronic states of single and double-layer gold might likewise be utilized in optical sensing units.
Electronic impacts likewise in the graphene layer
Another application concept arises from impacts triggered by the intercalated gold in the nearby graphene layer, which obviously depend upon the density of the gold. “A gold layer one atom thick causes an n-doping in the graphene. This means we obtain electrons as charge carriers,” states Forti. In areas where the gold is 2 atomic layers thick, precisely the opposite – p-doping – takes place. There, missing out on electrons or favorably charged so-called “holes” serve as charge providers. The gold likewise improves the interaction of plasmons (i.e. variations in the density of charge providers) with electro-magnetic radiation. “A structured, alternating arrangement of n- and p-doping in the graphene could thus be used. For example, as a highly sensitive yet high-resolution detector array for terahertz radiation like those used in materials testing, for security checks at airports, or for wireless data transmission.” states Starke.
Starke’s group has actually currently taken the next action in the production of two-dimensional rare-earth element layers. Also in an intercalation try out silver, a strictly crystalline two-dimensional silver layer formed in between silicon carbide and graphene. And what’s more: even this metal, which is normally an even much better electrical conductor than gold, ends up being a semiconductor when decreased to 2 measurements. The preliminary outcomes show that the energy needed to make the silver layer electrically conductive is most likely greater than for 2D gold. “The semiconductor properties of a component made from this material might therefore be thermally more stable than those of gold,” states Starke about possible useful repercussions.





