Energy-offering bacterial endosymbiont allows its unicellular eukaryotic host to breathe nitrate, showing that unicellular eukaryotes might get endosymbionts to enhance or change functions of their mitochondrial organelles.
Researchers from Bremen, together with their associates from the Max Planck Genome Center in Cologne and the water research study institute Eawag from Switzerland, have actually found a unique germs that lives inside a unicellular eukaryote and supplies it with energy. Unlike mitochondria, this so-called endosymbiont obtains energy from the respiration of nitrate, not oxygen. “Such partnership is completely new,” states Jana Milucka, the senior author on the Nature paper. “A symbiosis that is based on respiration and transfer of energy is to this date unprecedented.”
In basic, amongst eukaryotes, symbioses are rather typical. Eukaryotic hosts frequently co-exist with other organisms, such as germs. Some of the germs live inside the host cells or tissue, and carry out particular services, such as defense or nutrition. In return, the host supplies shelter and ideal living conditions for the symbiont. An endosymbiosis can even go that far that the germs loses its capability to make it through by itself outdoors its host.
This was likewise the case with the symbiosis found by the Bremen researchers in Lake Zug in Switzerland. “Our finding opens the possibility that simple unicellular eukaryotes, such as protists, can host energy-providing endosymbionts to complement or even replace the functions of their mitochondria,” states Jon Graf, very first author of the research study. “This protist has managed to survive without oxygen by teaming up with an endosymbiont capable of nitrate respiration.” The endosymbiont’s name ‘Candidatus Azoamicus ciliaticola’ shows this; a ‘nitrogen friend’ that stays within a ciliate.
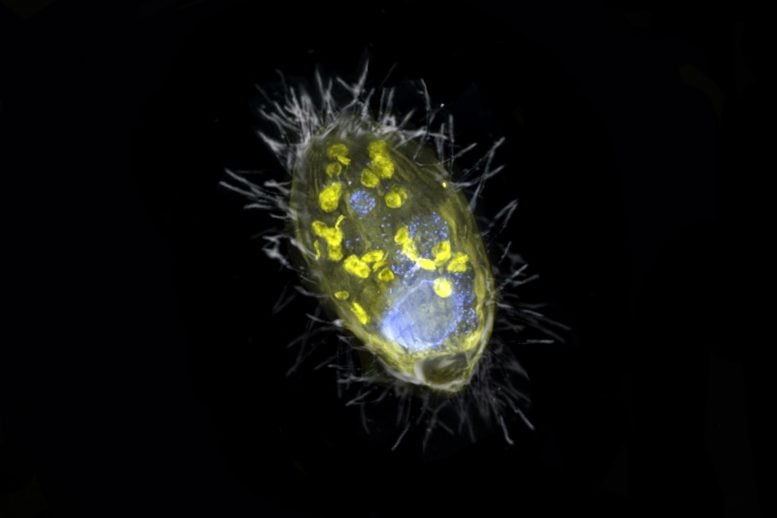
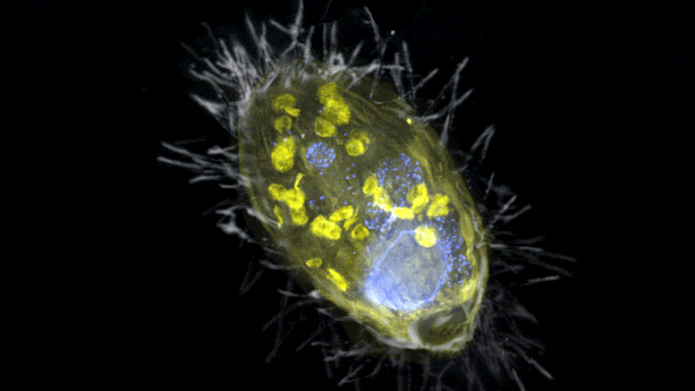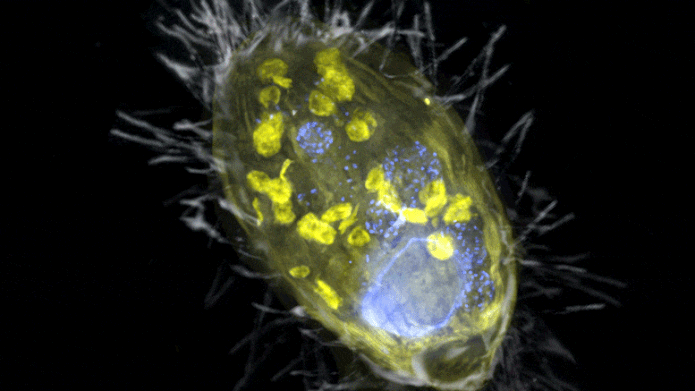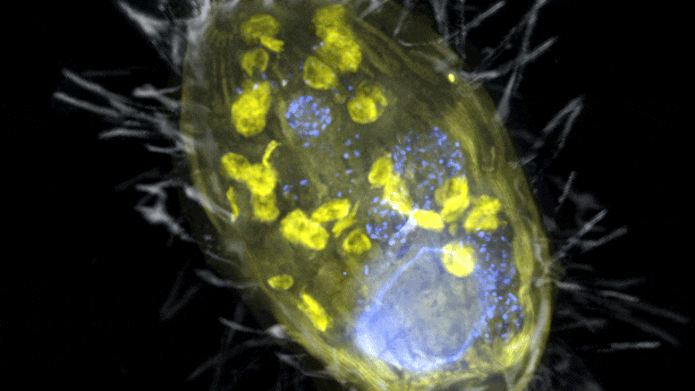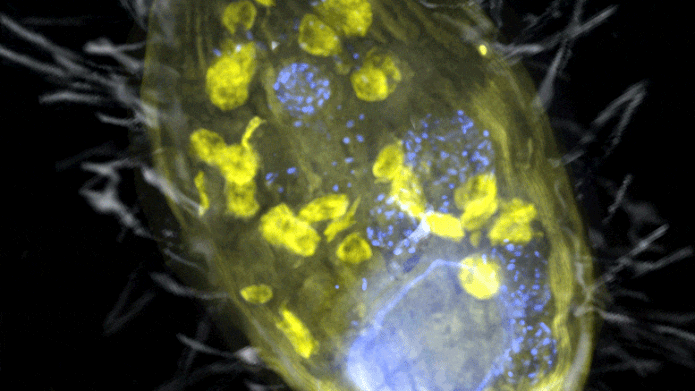
The figure is a composite of a scanning electron microscopic lense image (SEM, grey) and fluorescence images. Visible is the ‘Candidatus Azoamicus ciliaticola’ endosymbiont (envisioned by FISH, yellow) and bacterial victim in food vacuoles in addition to the big cell nucleus (stained by DAPI, blue). The external structure of the weakly fluorescent ciliate in addition to the cilia are likewise noticeable. Credit: Max Planck Institute for Marine Microbiology, S. Ahmerkamp
An intimate collaboration ends up being ever closer
So far, it has actually been presumed that eukaryotes in oxygen-free environments endure through fermentation, because mitochondria need oxygen in order to create energy. The fermentation procedure is well recorded and has actually been observed in numerous anaerobic ciliates. However, microbes cannot draw as much energy from fermentation, and they generally do not grow and divide as rapidly as their aerobic equivalents.
“Our ciliate has found a solution for this,” states Graf. “It has engulfed a bacterium with the ability to breathe nitrate and integrated it into its cell. We estimate that the assimilation took place at least 200 to 300 million years ago.” Since then, advancement has actually even more deepened this intimate collaboration.
Time-moved advancement
The advancement of mitochondria has actually continued in a comparable method. „All mitochondria have a typical origin,” discusses Jana Milucka. It is thought that more than a billion years back when an ancestral archaeon swallowed up a germs, these 2 began an extremely crucial symbiosis: this occasion marked the origin of the eukaryotic cell. Over time, the germs ended up being increasingly more incorporated into the cell, gradually minimizing its genome. Properties no longer required were lost and just the ones that benefitted the host were kept. Eventually, mitochondria progressed, as we understand them today. They have their own small genome in addition to a cell membrane, and exist as so-called organelles in eukaryotes. In the body, for instance, they exist in nearly every cell and provide them – and hence us – with energy.
“Our endosymbiont is capable of performing many mitochondrial functions, even though it does not share a common evolutionary origin with mitochondria,” states Milucka. “It is tempting to speculate that the symbiont might follow the same path as mitochondria, and eventually become an organelle.”
A possibility encounter
It is really incredible that this symbiosis has actually stayed unidentified for so long. Mitochondria work so well with oxygen – why shouldn’t there be a comparable for nitrate? One possible response is that nobody knew this possibility therefore nobody was searching for it. Studying endosymbioses is tough, as the majority of cooperative microbes cannot be grown in the lab. However, the current advances in metagenomic analyses have actually enabled us to acquire a much better insight into the intricate interaction in between hosts and symbionts. When evaluating a metagenome, researchers take a look at all genes in a sample. This technique is frequently utilized for ecological samples as the genes in a sample cannot be immediately designated to the organisms present. This indicates that researchers generally try to find particular gene series that pertain to their research study concern. Metagenomes frequently include countless various gene series and it is rather regular that just a little portion of them is examined in information.
Originally, the Bremen researchers were likewise searching for something else. The Research Group Greenhouse Gases at the Max-Planck-Institute for Marine Microbiology examines microbes associated with methane metabolic process. For this, they have actually been studying the deep-water layers of Lake Zug. The lake is extremely stratified, which indicates that there is no vertical exchange of water. The deep-water layers of Lake Zug hence have no contact with surface area water and are mostly separated. That is why they include no oxygen however are abundant in methane and nitrogen substances, such as nitrate. While searching for methane chewing germs with genes for nitrogen conversion, Graf discovered an astonishingly little gene series that encoded the total metabolic path for nitrate respiration. “We were all stunned by this finding and I began comparing the DNA with comparable gene series in a database,” states Graf. But the only comparable DNA came from that of symbionts that reside in aphids and other pests. “This didn’t make sense. How would insects get into these deep waters? And why?,” Graf keeps in mind. The researchers of the research study group began thinking video games and wagering.
Not alone in the dark lake
In completion, one believed dominated: The genome should come from a yet-unknown endosymbiont. To confirm this theory, members of the research study group carried out a number of explorations to Lake Zug in Switzerland. With the aid of the regional cooperation partner Eawag they gathered samples to look particularly for the organism which contains this distinct endosymbiont. In the laboratory, the researchers fished out numerous eukaryotes out of the water samples with a pipette. At last, utilizing a gene marker, it was possible to envision the endosymbiont and determine its protist host.
A last adventure one year back was expected to bring last certainty. It was a tough endeavor in the middle of winter season. Stormy weather condition, thick fog and time pressure due to very first news about Coronavirus in addition to a possible lockdown made the search in the huge lake a lot more tough. Nonetheless, the researchers prospered in recovering a number of samples from the deep water and bringing them to Bremen. These samples brought them last verification of their theory. “It is nice knowing that they are down there together,” states Jana Milucka. “Normally, these ciliates eat bacteria. But this one let one alive and partnered up with it.”
Many brand-new concerns
This finding provokes numerous amazing brand-new concerns. Are there comparable symbioses that have existed a lot longer and where the endosymbiont has currently crossed the border to an organelle? If such symbiosis exists for nitrate respiration, does it likewise exist for other substances? How did this symbiosis, which has existed for 200 to 300 million years, wind up in a post-glacial lake in the Alps that just formed 10,000 years ago? Moreover: “Now that we know what we are looking for, we found the endosymbiont’s gene sequences all around the world,” states Milucka. In France, in addition to in Taiwan, or in East African lakes that in part are much older than Lake Zug. Does the origin of this symbiosis depend on among them? Or did it begin in the ocean? These are the concerns that the research study group wishes to examine next.
Reference: “Anaerobic endosymbiont generates energy for ciliate host by denitrification” by Jon S. Graf, Sina Schorn, Katharina Kitzinger, Soeren Ahmerkamp, Christian Woehle, Bruno Huettel, Carsten J. Schubert, Marcel M. M. Kuypers and Jana Milucka, 3 March 2021, Nature.
DOI: 10.1038/s41586-021-03297-6