WHO Guideline Development Group: Currently no proof that it enhances survival and other essential steps.
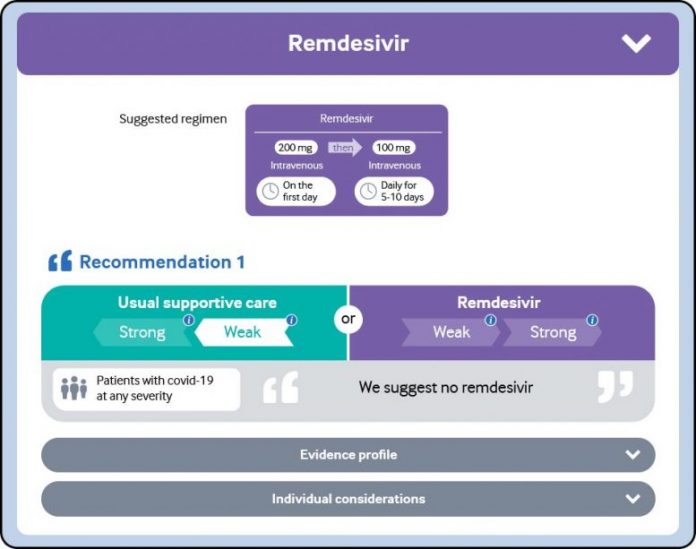
The antiviral drug remdesivir is not recommended for clients confessed to health center with covid-19, despite how badly ill they are, since there is presently no proof that it enhances survival or the requirement for ventilation, state a WHO Guideline Development Group (GDG) panel of global professionals in The BMJ today.
The suggestion becomes part of a living standard, established by the World Health Organization with the methodological assistance of MAGIC Evidence Ecosystem Foundation, to offer credible assistance on the management of covid-19 and assist medical professionals make much better choices with their clients.
Living standards work in fast-moving research study locations like covid-19 since they enable scientists to upgrade formerly vetted and peer-reviewed proof summaries as brand-new info appears.
Remdesivir has actually gotten around the world attention as a possibly reliable treatment for serious covid-19 and is progressively utilized to deal with clients in health center. But its function in medical practice has actually stayed unpredictable.
Today’s suggestion is based upon a brand-new proof evaluation comparing the results of a number of drug treatments for covid-19. It consists of information from 4 global randomized trials including over 7,000 clients hospitalized for covid-19.
After completely examining this proof, the WHO GDG professional panel, that includes professionals from worldwide consisting of 4 clients who have actually had covid-19, concluded that remdesivir has no significant result on death or on other essential results for clients, such as the requirement for mechanical ventilation or time to medical enhancement.
The panel acknowledged that the certainty of proof is low and stated the proof did not show that remdesivir has no advantage; rather, there is no proof based upon presently offered information that it does enhance essential client results.
But offered the staying possibility of essential damage, in addition to the fairly high expense and resource ramifications related to remdesivir (it should be offered intravenously), they evaluated this to be a suitable suggestion.
They likewise support ongoing enrolment into trials examining remdesivir, particularly to offer greater certainty of proof for particular groups of clients.
In a connected function short article, United States reporter Jeremy Hsu asks what now for remdesivir, considered that it is not likely to be the lifesaving drug for the masses that numerous have expected?
The complete story of remdesivir will not be understood till producer Gilead launches the complete medical research study reports, composes Hsu, however much will depend upon whether future research studies are created to check remdesivir’s prospective efficiency.
In the meantime, he states alternative treatments, such as the popular, low-cost, and extensively offered corticosteroid dexamethasone, that has actually been shown to lower death amongst badly ill covid-19 clients, are now affecting conversations about remdesivir’s cost-effectiveness.
Reference: “A living WHO guideline on drugs for covid-19” by Bram Rochwerg, et. al., 4 September 2020, BMJ.
DOI: 10.1136/bmj.m3379