A peaceful months-long legal battle in between the U.S. National Institutes of Health and drugmaker Moderna over COVID-19 vaccine patents just recently break into public view. The result of the fight has essential ramifications, not just for efforts to consist of the pandemic however more broadly for drugs and vaccines that might be important for future public health crises.
I teach drug guideline and patent law at Saint Louis University’s Center for Health Law Studies.
Moderna just recently provided to share ownership of its primary patent with the federal government to solve the disagreement. Whether or not this suffices to please the federal government’s claims, I think the disagreement indicates severe issues in the methods U.S. business bring drugs and vaccines to market.
United States was a significant funder of the Moderna vaccine
Vaccines have actually played an important function in the reaction to the pandemic.
In December 2020, Moderna ended up being the 2nd pharmaceutical business after Pfizer to acquire permission from the Food and Drug Administration to market a COVID-19 vaccine in the UnitedStates People have actually because grown so utilized to speaking about the “Moderna vaccine” that an important aspect in the history of how it was established threats being eclipsed: Moderna was not the sole designer of the vaccine.
Unlike a lot of the other pharmaceutical business associated with the COVID-19 vaccine race, Moderna is a beginner to drug and vaccine commercialization. Founded in Massachusetts in 2010, the business had actually never ever brought an item to market till the FDA licensed its COVID-19 vaccine in 2015.
Throughout the 2010 s, Moderna concentrated on the advancement of mRNA innovation, bring in over US$ 2 billion in financing from pharmaceutical business and other financiers. It went public in 2018.
Even prior to the pandemic, research study on both coronaviruses and vaccine prospects versus emerging pathogens was a concern for companies running in the general public health area. In 2015, the National Institute of Allergy and Infectious Diseases, an institute within the NIH, signed a cooperative R&D contract with Moderna on standard research study, consisting of the advancement of brand-new vaccines. The contract led to a concealed quantity of financing and support with research study.
In addition, after the COVID-19 break out started Moderna likewise got practically $1 billion in financing from the Biomedical Advanced Research and Development Authority, which runs within the Department of Health and HumanServices This financing was particularly targeted to the advancement of a COVID-19 vaccine prospect.
Researchers have actually computed that, jointly, the U.S. federal government has actually offered $2.5 billion towards the advancement and commercialization of Moderna’s COVID-19 vaccine.
United States, Moderna researchers working side by side
In addition to offering financial backing, the federal government contributed in the advancement of Moderna’s vaccine for other factors. Namely, federal researchers worked along with Moderna researchers on various elements of the vaccine.
These contributions consisted of dealing with dosing systems, and the NIH stated federal researchers produced the supported spike proteins that are a crucial part of the vaccine made by Moderna.
The value of the function played by federal researchers in their deal with Moderna would quickly emerge. A 2019 contract with a 3rd party clearly acknowledged this, mentioning mRNA vaccine prospects “developed and jointly owned by NIAID and Moderna.” And by late 2020, the U.S. federal government was calling it the “NIH-Moderna COVID-19 vaccine.”
While the U.S. federal government has actually invested cash on COVID-19 vaccines made by other business, its close participation in the R&D phases of Moderna’s sets it apart.
How it ended up being a patent disagreement
As advancement of the vaccine advanced, Moderna requested a number of patents, every one covering various elements of the vaccine. U.S. law enables developers to get patents on items or approaches that are brand-new, not apparent, and helpful. While some early modern-day vaccines– like the polio vaccine established by Jonas Salk’s group– were not covered by patents, from the late 20 th century onward it ended up being really typical for one or numerous patents to cover a freshly established vaccine.
In requesting some patents connected to its vaccine, Moderna called National Institute of Allergy and Infectious Diseases researchers as co-inventors along with Moderna researchers. This held true, for instance, in a patent application dated May 2020 for a fairly small part of the vaccine.
However, in July 2021, Moderna made it clear that it would not call federal government researchers as co-inventors in a patent application covering a a lot more substantial part of the vaccine: the mRNA series utilized to produce the vaccine, called mRNA-1273
Moderna’s position was that Moderna researchers alone had actually picked the series. The business notified the Patent and Trademark Office of its position in a 2020 declaration.
In November 2021, federal government authorities openly challenged the business’s choice after months of stopped working settlements with the business. Moderna then took to social media to protect its position, tweeting:
“Just because someone is an inventor on one patent application relating to our COVID-19 vaccine does not mean they are an inventor on every patent application relating to the vaccine.”
Our main declaration on copyright. Thread.
— Moderna (@moderna_tx) November 11, 2021
By contrast, the National Institutes of Health argued that 3 NIAID researchers– Kizzmekia Corbett, Barney Graham and John Mascola– had actually meaningfully added to the creation, though they have actually decreased to openly define how. If real, patent law states they need to be called co-inventors.
But this disagreement is not simply about clinical concepts or technical elements of the law. While patents are likewise considered as proxies for determining clinical credibility, their most instant and effective impact is to offer patent holders a substantial quantity of control over the covered innovation– in this case, the primary part of the vaccine made by Moderna.
From an useful viewpoint, omitting federal researchers from the application suggests that Moderna alone gets to choose how to utilize the vaccine, whether to accredit it and to whom. If, by contrast, the federal government co-owns the vaccine, federal patent law enables each of the joint owners to take part in a range of actions– from making and offering the vaccine to licensing it– without the authorization of the other owners.
This is particularly appropriate in cases of item deficiency or possible rates problems in connection with the commercialization of the vaccine. For circumstances, the U.S. would have the capability to enable more producers to produce vaccines utilizing the mRNA-1273 innovation. In addition, it might direct vaccine dosages anywhere it likes, consisting of to lower-income nations that have actually gotten couple of vaccines up until now.
Broader ramifications
The continuous fight in between the federal government and an emerging star in the pharmaceutical market is yet another episode in a complex relationship in between stars with complementary yet unique functions in the production of drugs and vaccines.
On the one hand, the federal government has actually long played a vital function in both carrying out and moneying standard research study. On the other, it does not have the resources and capability to bring most kinds of brand-new drugs and vaccines to market by itself.
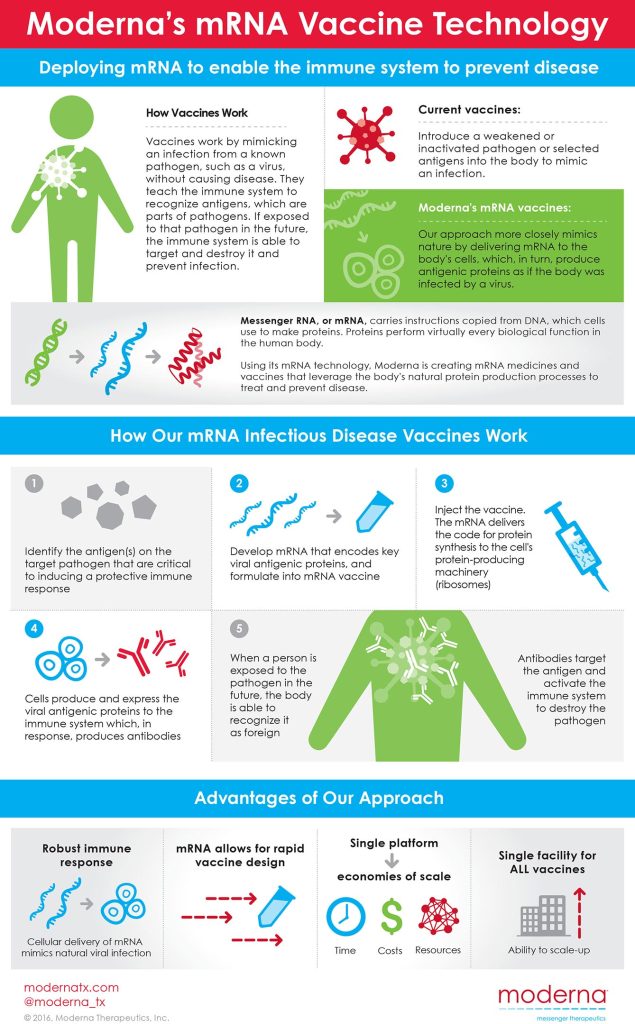
Moderna discusses its mRNA innovation.
The pharmaceutical market hence plays a crucial and essential function in drug development, which I think need to be rewarded– although not boundlessly.
If the NIH is appropriate about co-ownership of the vaccine, then Moderna is unduly utilizing a legal tool to accomplish a position of market control– a benefit it does not be worthy of. This position of sole control ends up being a lot more troublesome due to the substantial quantities of public cash that moneyed the advancement of this vaccine. This balance out a few of Moderna’s monetary danger, even as the business tasks to make $15 billion to $18 billion in profits from vaccine sales in 2021 alone, with a lot more anticipated in 2022.
However, even if the NIH dominates in the patent disagreement, it is necessary to comprehend the restrictions of such a “win.” The U.S. would remain in a position to accredit the vaccine, for instance, and might do so by needing that licensees consent to fair circulation of vaccine dosages.
But co-ownership would not allow the federal government to repair any of the other issues that presently impact the production and circulation of COVID-19 vaccines, such as scaling up production or structure facilities to provide vaccine dosages.
In my view, the disagreement is a pointer of the numerous issues embedded in how vaccines are made and provided in the U.S. And it reveals that when taxpayers fund standard research study of a drug, they should have more of the control– and benefits– when that drug prospers.
Written by Ana Santos Rutschman, Assistant Professor of Law, Saint Louis University.
This short article was very first released in The Conversation.![]()