Research on φX174, a bacteriophage, reveals possible in dealing with antibiotic-resistant infections. Historically substantial in phage treatment and molecular biology, φX174’s distinct cell-lysing system might lead the way for ingenious prescription antibiotics.
φX174, a bacteriophage studied for its capacity in combating antibiotic-resistant germs, provides brand-new insights into establishing alternative prescription antibiotics.
In the age of < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>COVID-19</div><div class=glossaryItemBody>First identified in 2019 in Wuhan, China, COVID-19, or Coronavirus disease 2019, (which was originally called "2019 novel coronavirus" or 2019-nCoV) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has spread globally, resulting in the 2019–22 coronavirus pandemic.</div>" data-gt-translate-attributes="(** )" tabindex ="0" function ="link" > COVID-19, the word”< period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>virus</div><div class=glossaryItemBody>A virus is a tiny infectious agent that is not considered a living organism. It consists of genetic material, either DNA or RNA, that is surrounded by a protein coat called a capsid. Some viruses also have an outer envelope made up of lipids that surrounds the capsid. Viruses can infect a wide range of organisms, including humans, animals, plants, and even bacteria. They rely on host cells to replicate and multiply, hijacking the cell's machinery to make copies of themselves. This process can cause damage to the host cell and lead to various diseases, ranging from mild to severe. Common viral infections include the flu, colds, HIV, and COVID-19. Vaccines and antiviral medications can help prevent and treat viral infections.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" > infection (********************** )” stimulates ideas of contagion, illness, and even death.But what if there were an infection– an extremely small infection efficient in duplicating itself numerous times every half hour– that could treat a serious bacterial infection resistant to all understood prescription antibiotics?It is this hope that encouragesBilClemons, theArthur andMarianHanischMemorial(*********************************************************************************************************************************************************************************************************************************************** )ofBiochemistry, to look into the infection called φX174
Understanding φX174
φX174 is a bacteriophage or, more merely, a phage: an infection that targets bacterial cells.From a human viewpoint, φX174 leads a basic life:It discovers its host germs, parks on its surface area, injects a hair of< period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip =(***************************************************************************** )data-gt-translate-attributes=" (** )" tabindex ="0" function ="link" > DNA into the bacterial cell, duplicates its DNA over and over once again, requires the cell to make viral proteins, puts together the DNA and protein into brand-new virions( copies of the phage), and after that burst the cell wall of the germs so the virions can discover other hosts to contaminate.
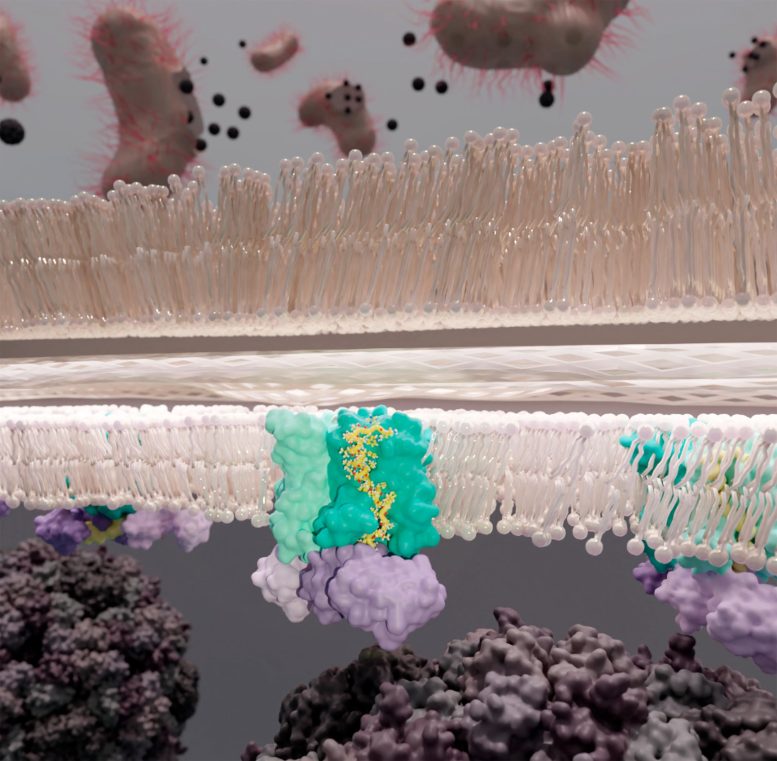
The escape of ΦX 174 from its bacterial host.In the membrane is the YES complex( E. coli enzymeMra Y[cyan], phage protein E[yellow], & E. coli chaperone SlyD[purple]) where protein E interrupts peptidoglycan synthesis by hinderingMra Y permitting breaching of the cell wall[tan]Credit:KarenOrta
It is this escape system that theClemons group clarifies in their paper just recently released in the journalScience,“The mechanism of the phage encoded protein antibiotic from φX174.”Relying on images from single-particle electron cryomicroscopy, it is exposed that φX174’s E protein accompanies its bacterial host’s proteins Mra Y and SlyD to form a steady complex– the YES complex. This leads to cell lysis: the breaching of the bacterial cell wall and the death of the germs.
Historical Context of Phage Discovery
φX174 has actually been on researchers’ radar for about 100 years. In the early 20 th century, the presence of phages was just thought. Working individually, British bacteriologist Frederick Twort and Quebecois researcher Félix d’Herelle postulated the presence of phages based upon the habits of bacterial cultures in their labs. Sometimes, when the germs were expected to be multiplying on their Petri meals, shiny spots– plaques– would appear where no germs grew. Passing these samples through filters recorded the germs while permitting their small unnoticeable killers to go through. Whatever it was that effectively moved through the filters, it was too little to be seen with a microscopic lense.
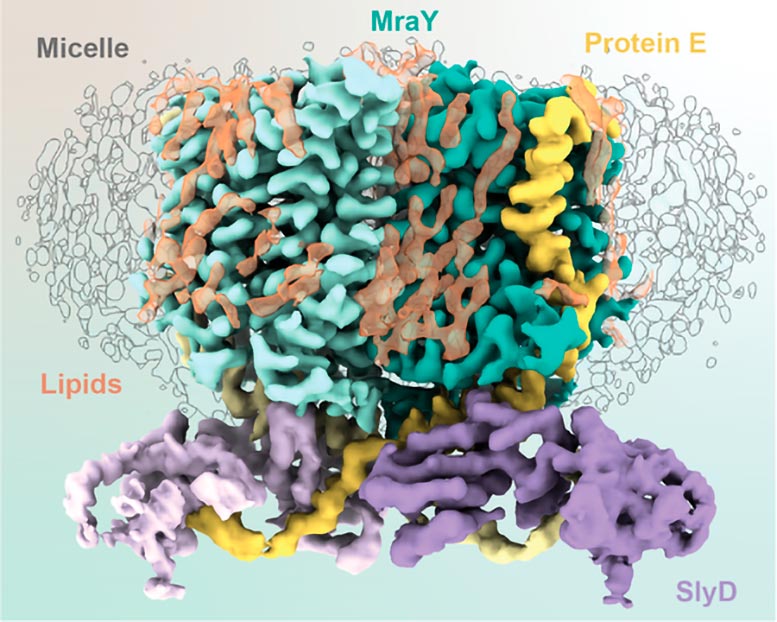
Schematic of the components making up the YES complex. Credit: Karen Orta
D’Herelle, working in Paris in 1917, recommended that these killers should be bacteria-eating infections and was all set to check this theory. According to urban myth, as Clemons relates it, d’Herelle filtered sewage water consistently and after that consumed it to see if it was safe to take in. He felt himself to be unscathed, so he used a sip to his laboratory assistant, who was similarly the same. D’Herelle then provided the filtered sewage water to a client, a young kid with serious dysentery who was on the verge of death. With this phage mixed drink, which more than likely consisted of φX174, the young boy was rapidly brought back to health.
Researchers from throughout Europe pertained to Paris to deal with d’Herelle One such scientist, Croatian microbiologist Vladimir Serti č, invested a years operating in d’Herelle’s laboratory. It was Serti č and his assistant, Nikolai Boulgakov, who created a taxonomy for recognized phages. φX174’s unique sounding name, in Sertic’s category plan, merely suggests “the 174 th infection in the tenth [roman numeral X] series of phages that target several germs,” of the class φ: phages that act versus several germs. Phage treatment continued to treat bacterial illness, however it eliminated too, most likely since scientists did not yet understand how to cleanse the by-products of phage duplication such as bacterial particles, which can be harmful.
Evolution of Phage Therapy and Research
Phage research study and treatment ended up being fragmented under the pressure of World War II. For the Western allies, the production of extremely reliable penicillin totally eclipsed phage treatment, ending up being the particular service for bacterial infections. Penicillin was a military trick not shown the Eastern allies or the Axis powers, so Soviet physicians continued the restorative usage of phages, a practice that continues today in the nations of the previous Soviet Union.
Although phages fell out of favor with medical scientists in Western nations in the years after World War II, research study researchers ended up being amazed with them. φX174, although just one of billions of various kinds of phages, relocated to the front of the line as a beneficial speculative tool for the establishing field of molecular biology.
Robert L. Sinsheimer, teacher of biophysics at Caltech from 1957 to 1977, contributed in establishing φX174 as a design organism. His laboratory carried out the mapping of φX174’s genome and found a lot of its more interesting functions. As Sinsheimer informed the story in a 1991 narrative history interview, he welcomed Max Delbr ück, teacher of biology at Caltech, to provide a series of talks at Iowa State University in the early 1950 s where Sinsheimer was then on the professors. “He [Delbrück] simply blew us away with his phage work,” Sinsheimer stated. “It was absolutely glorious.”

Max Delbr ück with phage group,1949 Left to right: Jean Jacques Weigle, Ole Maaloe, Elie Wollman, Gunther S. Stent, Max Delbr ück, and G.Soli Credit: Ross Madden, BlackStar 1949-02, 1.3.1-16, CaltechPhotographs California Institute of Technology Archives and Special Collections
Delbr ück, who had actually at first trained as a physicist at the University of Göttingen before the war, was constructing a cadre of phage scientists at Caltech and utilizing the infections to penetrate the secrets of molecular genes. Sinsheimer made it his objective to come to Caltech throughout a six-month leave in 1953 to discover how to deal with phages. One day while being in Delbr ück’s workplace talking about how to continue with virology, the 2 guys concluded that it may be helpful to study the tiniest and possibly most basic phages to much better comprehend viral structure and duplication. Sinsheimer evaluated phage prospects, decided on φX174, obtained samples from laboratories in England and France, and set to work.
There started a string of firsts for science based upon φX174 In a 1966 essay, Sinsheimer described φX174 as “multum in parvo”: Latin for “much in little.” Throughout the 1950 s and 1960 s, φX174 continuously shocked scientists. In 1959, 2 years after signing up with Caltech, Sinsheimer identified that φX174 had just a single hair of DNA that it injected into the host cell to start duplication. This was a surprise considered that DNA had actually been found to have a double-helical structure just a couple of years previously. In 1962, Sinsheimer hypothesized that φX174’s DNA was formed like a circular ring, something molecular biologists had actually not yet envisioned. In 1977, Frederick Sanger of the University of Cambridge was the very first individual to totally series a genome, making him the 1980 Nobel Prize inChemistry That genome came from φX174 The phage itself was obtained from Sinsheimer.

Robert Sinsheimer in his lab,1974 Credit: FloydClark 10.24-116, CaltechPhotographs California Institute of Technology Archives and Special Collections
By the late 1970 s, much of the life process of φX174 was well comprehended, however unpredictabilities stayed. It was presumed that φX174 broke out of its bacterial host by obstructing the synthesis of the peptidoglycan layer– a crucial protective barrier in the cell wall of all germs– simply as penicillin and other pharmaceutical prescription antibiotics do.
For most phages, researchers had actually discovered how they make specialized enzymes, endolysins, that break down the sugar– amino < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>acid</div><div class=glossaryItemBody>Any substance that when dissolved in water, gives a pH less than 7.0, or donates a hydrogen ion.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" > acid polymer that comprises the peptidoglycan layer.But these enzymes are too big to be consisted of in the DNA of a small phage like φX174
ModernResearch on φX174
“The φX174 genome is really small,”Clemons discusses.“If you were to encode something that achieves cell lysis in the way a lysozyme does—an enzyme found in our tears and saliva that provide protection against bacteria by mimicking endolysins—there would be no room for other proteins on the φX174 genome. φX174 is part of a group of these viruses that are too small to have complex lysis machinery, so these phages had to evolve very simple ways of lysing bacterial cells.”
Different phages and prescription antibiotics disrupt the synthesis of peptidoglycan at differing points while doing so.The E protein of φX174 targetsMra Y, a membrane enzyme that catalyzes the synthesis of a peptidoglycan precursor.To total its harmful work, φX174’s protein E needs another protein, SlyD, which it pirates from its bacterial host.“It’s a mystery,” statesClemons,(********************************************************** )(**************** )
These 3 representatives, one viral and 2 from the host, consist of the YES complex:Mra Y , protein E, S lyD.Essentially, the E protein of φX174 braids itself withMra Y, hinderingMra Y’s enzymatic activity. SlyD binds and supports the protein E andMra Y complex without callingMra Y.
Implications forFutureAntibioticDevelopment
This discovery stands poised to assist scientists meet bacteriophages’ preliminary guarantee as an antibiotic restorative.Antibiotics have actually conserved numerous varieties of lives over the previous century, however the innovation of brand-new classes of prescription antibiotics has actually been not able to stay up to date with the capability of germs to establish resistance to them. Bacteria likewise alter to withstand phages, however unlike pharmaceutical prescription antibiotics that need substantial human effort to enhance their structure, phages themselves can alter, countering brand-new bacterial defenses. We cope with a significant variety of phages in our bodies, lots of numerous trillions. It is the hope of Clemons and other scientists in the field that marshaling the best phages at the correct time to deal with bacterial infections might develop a brand-new, more long lasting antibiotic, one we significantly require as we challenge antibiotic resistant germs.
Reference: “The mechanism of the phage-encoded protein antibiotic from ΦX174” by Anna K. Orta, Nadia Riera, Yancheng E. Li, Shiho Tanaka, Hyun Gi Yun, Lada Klaic and William M. Clemons Jr., 14 July 2023, Science
DOI: 10.1126/ science.adg9091
Co- authors of “The mechanism of the phage encoded protein antibiotic from φX174” consist of Anna K. Orta, Nadia Riera, Evelyn Yancheng Li, Shiho Tanaka, Hyun Gi Yun, and LadaKlaic Funding was offered by the < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>National Institutes of Health</div><div class=glossaryItemBody>The National Institutes of Health (NIH) is the primary agency of the United States government responsible for biomedical and public health research. Founded in 1887, it is a part of the U.S. Department of Health and Human Services. The NIH conducts its own scientific research through its Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program. With 27 different institutes and centers under its umbrella, the NIH covers a broad spectrum of health-related research, including specific diseases, population health, clinical research, and fundamental biological processes. Its mission is to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" >NationalInstitutes ofHealth and the G.Harold andLeila Y.MathersFoundation





