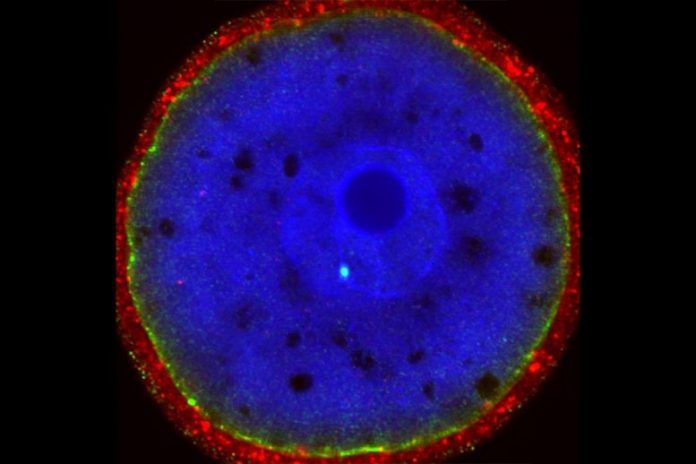
Staining for RAD51 (brilliant cyan-colored dot) in a fertilized one-cell mouse embryo reveals repair work of a CRISPR-induced DNA break. Credit: Image thanks to the scientists.
Novel approach, established by McGovern Institute scientists, might cause much safer, more effective gene treatments.
Gene modifying, or actively altering a gene’s DNA series, is an effective tool for studying how anomalies trigger illness, and for making modifications in a person’s DNA for healing functions. An unique approach of gene modifying that can be utilized for both functions has actually now been established by a group led by Guoping Feng, the James W. (1963) and Patricia T. Poitras Professor in Brain and Cognitive Sciences at MIT.
“This technical advance can accelerate the production of disease models in animals and, critically, opens up a brand-new methodology for correcting disease-causing mutations,” states Feng, who is likewise a member of the Broad Institute of Harvard and MIT and the associate director of the McGovern Institute for Brain Research at MIT. The brand-new findings were released online on May 26, 2021, in the journal Cell.
Genetic designs of illness
A significant objective of the Feng laboratory is to specifically specify what fails in neurodevelopmental and neuropsychiatric conditions by engineering animal designs that bring the gene anomalies that trigger these conditions in people. New designs can be created by injecting embryos with gene modifying tools, together with a piece of DNA bring the wanted anomaly.
In one such approach, the gene modifying tool CRISPR is set to cut a targeted gene, thus triggering natural DNA systems that “repair” the damaged gene with the injected design template DNA. The crafted cells are then utilized to produce offspring efficient in passing the hereditary modification on to additional generations, developing a steady hereditary line in which the illness, and treatments, are evaluated.
Although CRISPR has actually sped up the procedure of creating such illness designs, the procedure can still take months or years. Reasons for the ineffectiveness are that lots of cured cells do not go through the wanted DNA series modification at all, and the modification just takes place on among the 2 gene copies (for a lot of genes, each cell includes 2 variations, one from the daddy and one from the mom).
In an effort to increase the performance of the gene modifying procedure, the Feng laboratory group at first assumed that including a DNA repair work protein called RAD51 to a basic mix of CRISPR gene modifying tools would increase the opportunities that a cell (in this case a fertilized mouse egg, or one-cell embryo) would go through the wanted hereditary modification.
As a test case, they determined the rate at which they had the ability to insert (“knock-in”) an anomaly in the gene Chd2 that is connected with autism. The total percentage of embryos that were properly modified stayed the same, however to their surprise, a considerably greater portion brought the wanted gene modify on both chromosomes. Tests with a various gene yielded the very same unanticipated result.
“Editing of both chromosomes simultaneously is normally very uncommon,” describes postdoc Jonathan Wilde. “The high rate of modifying seen with RAD51 was truly striking, and what began as a basic effort to make mutant Chd2 mice rapidly developed into a much larger task concentrated on RAD51 and its applications in genome modifying,” states Wilde, who co-authored the Cell paper with research study researcher Tomomi Aida.
A molecular photocopier
The Feng laboratory group next set out to comprehend the system by which RAD51 boosts gene modifying. They assumed that RAD51 engages a procedure called interhomolog repair work (IHR), where a DNA break on one chromosome is fixed utilizing the 2nd copy of the chromosome (from the other moms and dad) as the design template.
To test this, they injected mouse embryos with RAD51 and CRISPR however neglected the design template DNA. They set CRISPR to cut just the gene series on among the chromosomes, and after that evaluated whether it was fixed to match the series on the uncut chromosome. For this experiment, they needed to utilize mice in which the series on the maternal and paternal chromosomes were various.
They discovered that control embryos injected with CRISPR alone seldom revealed IHR repair work. However, addition of RAD51 substantially increased the variety of embryos in which the CRISPR-targeted gene was modified to match the uncut chromosome.
“Previous studies of IHR found that it is incredibly inefficient in most cells,” states Wilde. “Our finding that it occurs much more readily in embryonic cells and can be enhanced by RAD51 suggest that a deeper understanding of what makes the embryo permissive to this type of DNA repair could help us design safer and more efficient gene therapies.”
A brand-new method to remedy disease-causing anomalies
Standard gene treatment techniques that count on injecting a restorative piece of DNA to function as a design template for fixing the anomaly engage a procedure called homology-directed repair work (HDR).
“HDR-based strategies still suffer from low efficiency and carry the risk of unwanted integration of donor DNA throughout the genome,” describes Feng. “IHR has the potential to overcome these problems because it relies upon natural cellular pathways and the patient’s own normal chromosome for correction of the deleterious mutation.”
Feng’s group went on to recognize extra DNA repair-associated proteins that can promote IHR, consisting of a number of that not just promote high levels of IHR, however likewise quelch mistakes in the DNA repair work procedure. Additional experiments that permitted the group to analyze the genomic functions of IHR occasions offered much deeper insight into the system of IHR and recommended manner ins which the method can be utilized to make gene treatments much safer.
“While there is still a great deal to learn about this new application of IHR, our findings are the foundation for a new gene therapy approach that could help solve some of the big problems with current approaches,” states Aida.
Reference: “Efficient embryonic homozygous gene conversion via RAD51-enhanced interhomolog repair” by Jonathan J. Wilde, Tomomi Aida, Ricardo C.H. del Rosario, Tobias Kaiser, Peimin Qi, Martin Wienisch, Qiangge Zhang, Steven Colvin and Guoping Feng, 26 May 2021, Cell.
DOI: 10.1016/j.cell.2021.04.035
This research study was supported by the Hock E. Tan and K. Lisa Yang Center for Autism Research at MIT, the Poitras Center for Psychiatric Disorders Research at MIT, an NIH/NIMH Conte Center Grant, and the NIH Office of the Director.