Illustration of the communicating thick and thin filaments in the heart sarcomere based upon structural cryo electron-tomography information. Credit: MPI of Molecular Physiology
Scientists caught the very first true-to-life 3D picture of the thick filament in mammalian heart muscle.
Atrial fibrillation, cardiac arrest, and stroke are amongst the extreme health conditions that can come from hypertrophic cardiomyopathy, an important consider abrupt heart death amongst people under 35 years of ages.
“The heart muscle is a central engine of the human body. Of course, it is easier to fix a broken engine, if you know how it is built and how it functions,” states StefanRaunser “At the beginning of our muscle research, we have successfully visualized the structure of the essential muscle building blocks and how they interact using electron cryo-microscopy.”
“However, these were static images of proteins taken out of the living cell. They only tell us little about how the highly variable, dynamic interplay of muscle components moves the muscle in its native environment,” states Raunser.
Through thick and thin
Skeletal and heart muscles agreement upon the interaction of 2 kinds of parallel protein filaments in the sarcomere: thin and thick. The sarcomere is partitioned in numerous areas, called zones and bands, in which these filaments are set up in various methods.
The thin filament includes F-actin, troponin, tropomyosin, and nebulin. The thick filament is formed of myosin, titin, and myosin-binding protein C (MyBP-C). The latter can form links in between the filaments, whereas myosin, the so-called motor protein engages with the thin filament to produce force and contraction.
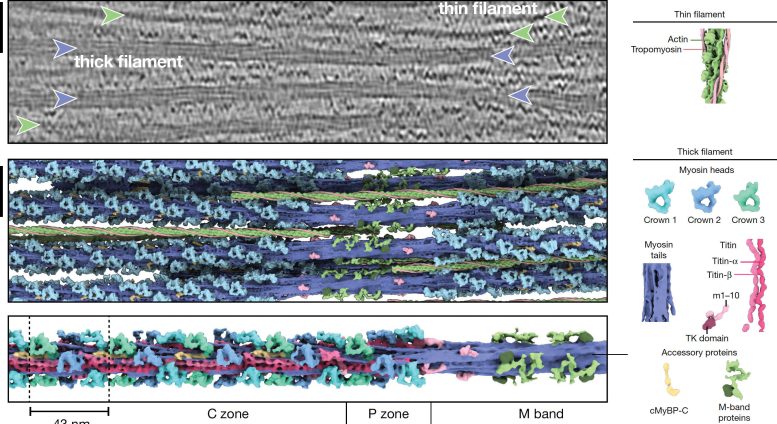
Thick filament structure in the unwinded heart sarcomere. The upper image reveals a tomographic piece of a heart sarcomere. Thin filaments are marked with green and thick filaments with a purple arrow. The middle image reveals the rebuilt thick (purple) and thin (green) filaments. The lower image reveals the structure of the thin filament covering throughout numerous sarcomere areas. The scale bar programs 50 nm. Credit:
MPI of Molecular Physiology
Alterations in the thick filament proteins are connected with muscle illness. A comprehensive photo of the thick filament would be of tremendous significance for establishing therapeutical methods to treat these illness, however has actually been missing out on up until now.
Milestones in muscle research study
“If you want to fully understand how the muscle works on the molecular level, you need to picture its components in their natural environment – one of the biggest challenges in biological research nowadays that cannot be tackled by traditional experimental approaches,” states Raunser.
To conquer this challenge his group established an electron cryo-tomography workflow particularly customized to the examination of muscle samples: The researchers flash-freeze mammalian heart muscle samples, produced by the Gautel group in London, at an extremely low temperature level (- 175 ° C).
https://www.youtube.com/watch?v=kBIL82 PTMt4
3D structure of the sarcomere revealing thick (purple) and thin (green) filaments. Credit: MPI of Molecular Physiology
This protects their hydration and great structure and hence their native state. A focused ion beam (FIB milling) is then used to thin out the samples to a perfect density of around 100 nanometers for the transmission electron microscopic lense, which gets numerous images as the sample is slanted along an axis. Finally, computational approaches rebuild a three-dimensional image at high resolution.
In current years, Raunser’s group effectively used the personalized workflow, leading to 2 current groundbreaking publications: They produced the very first high-resolution pictures of the sarcomere and of an up until now ambiguous muscle protein called nebulin. Both research studies supply extraordinary insights into the 3D company of muscle proteins in the sarcomere, e. g. how myosin binds to actin to manage contraction and how nebulin binds to actin to support it and to identify its length.
Completing the painting
In their present research study, the researchers produced the very first high-resolution picture of the heart thick filament covering throughout numerous areas in the sarcomere. “With 500 nm length this makes for the longest and biggest structure ever resolved by cryo-ET,” states Davide Tamborrini from the MPI Dortmund, first-author of the research study.
Even more outstanding are the freshly acquired insights into the thick filament’s molecular company and hence into its function. The plan of the myosin particles depends upon their position in the filament.
The researchers believe, that this enables the thick filament to sense and procedure various muscle-regulating signals and hence to manage the strength of contraction depending upon the sarcomere area. They likewise exposed how titin chains run along the filament. Titin chains link with myosin, functioning as a scaffold for its assembly and most likely managing a length-depending activation of the sarcomere.
“Our aim is to paint a complete picture of the sarcomere one day. The image of the thick filament in this study is ‘only’ a snapshot in the relaxed state of the muscle. To fully understand how the sarcomere functions and how it is regulated, we want to analyze it in different states e. g. during contraction,” states Raunser.
Comparison with samples from clients with muscle illness will eventually add to a much better understanding of illness like hypertrophic cardiomyopathy and to the advancement of ingenious treatments.
Reference: “Structure of the native myosin filament in the relaxed cardiac sarcomere” by Davide Tamborrini, Zhexin Wang, Thorsten Wagner, Sebastian Tacke, Markus Stabrin, Michael Grange, Ay Lin Kho, Martin Rees, Pauline Bennett, Mathias Gautel and Stefan Raunser, 32 October 2023, Nature
DOI: 10.1038/ s41586-023-06690 -5





