This color-enhanced image, taken by scanning electron microscopy, reveals big amounts of SARS-CoV-2 particles (purple) that have break out of kidney cells (green), which the infection pirated for duplication. The bulging, round cells in the upper-right and bottom-left corners are distorted and ready to rupture from the viral particles within, and are starting to self-destruct. Credit: NIAID Integrated Research Facility
Scientists team up to design the complex protein accountable for SARS-CoV-2 duplication, exposing its possible vulnerable points for drug advancement.
In February 2020, a trio of bio-imaging specialists were sitting amiably around a table at a clinical conference in Washington, D.C., when the discussion moved to what was then a distressing viral epidemic in China. Without visualizing the worldwide catastrophe to come, they questioned aloud how they may contribute.
Nearly a year and a half later on, those 3 researchers and their lots of partners throughout 3 nationwide labs have actually released a detailed research study in Biophysical Journal that – along with other current, complementary research studies of coronavirus proteins and genes – represents the initial step towards establishing treatments for that viral infection, now scorched into the worldwide awareness as COVID-19.
Their fundamental work concentrated on the protein-based device that makes it possible for the SARS-CoV-2 infection to pirate our own cells’ molecular equipment in order to reproduce inside our bodies.
From structure to operate to services
“It has actually been said that all organisms are simply a method for DNA to make copies of itself, and no place is this truer than when it comes to an infection,” stated Greg Hura, a personnel researcher at Lawrence Berkeley National Laboratory (Berkeley Lab) and among the research study’s lead authors. “A virus’s singular task is to make copies of its genetic material – unfortunately, at our expense.”
Viruses and mammals, consisting of people, have actually been stuck in this fight for countless years, he included, and over that time the infections have actually developed lots of techniques to get their genes copied inside us, while our bodies have actually developed counter defenses. And although infections typically carry out a long list of other activities, their capability to damage us with an infection actually does boil down to whether they can reproduce their hereditary product (either RNA or DNA, depending upon the types) to make more viral particles, and utilize our cells to equate their hereditary code into proteins.
The protein-based device accountable for RNA duplication and translation in coronaviruses – and lots of other infections – is called the RNA transcription complex (RTC), and it is a really powerful piece of biological weapons.
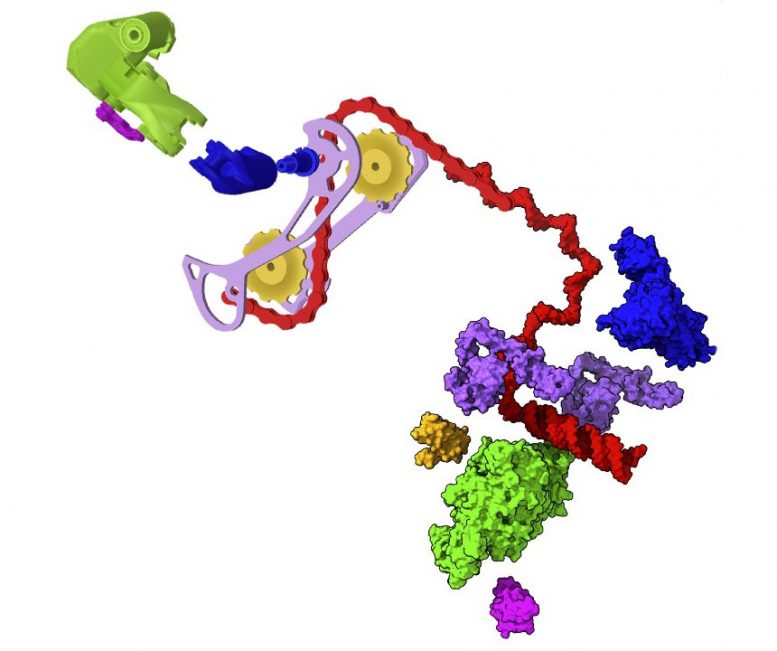
A making of the SARS-CoV-2 equipment showing its capability to quickly move structural plan – like a bike altering equipments – in order to carry out various jobs. Credit: Greg Hura/Berkeley Lab
To effectively replicate viral RNA for brand-new infection particles and produce the brand-new particles’ lots of proteins, the RTC should: compare viral and host RNA, acknowledge and match RNA bases rather of extremely comparable DNA bases that are likewise plentiful in human cells, transform their RNA into mRNA (to fool human ribosomes into equating viral proteins), user interface with copy error-checking particles, and transcribe particular areas of viral RNA to magnify particular proteins over others depending upon requirement – while at all times attempting to avert the host body immune system that will acknowledge it as a foreign protein.
As astonishing as this sounds, any freshly developed infection that achieves success “must have machines that are incredibly sophisticated to overcome mechanisms we have evolved,” described Hura, who heads the Structural Biology department in Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging Division.
He and the other research study leads – Andrzej Joachimiak of Argonne National Laboratory and Hugh M. O’Neill at Oak Ridge National Laboratory – concentrate on exposing the atomic structure of proteins in order to comprehend how they operate at the molecular level. So, the trio understood from the minute they initially went over COVID-19 at the table that studying the RTC would be especially difficult due to the fact that multitasking protein makers like the RTC aren’t fixed or stiff, as molecular diagrams or ball-and-stick designs may recommend. They’re versatile and have actually associated particles, called nonstructural and accessory proteins (Nsps), that exist in a plethora of quickly reorganizing types depending upon the job at hand – comparable to how an equipment shifter on a bike rapidly adjusts the car to altering surface.
Each of these Nsp plans provide insights into the protein’s various activities, and they likewise expose various parts of the general RTC surface area, which can be analyzed to discover locations where possible drug particles might bind and prevent the whole device.
So, following their serendipitous merging in Washington, the trio hatched a strategy to pool their understanding and nationwide laboratory resources in order to record the structure of as lots of RTC plans as possible, and determine how these types communicate with other viral and human particles.
Science throughout shutdowns
The examination depended upon integrating information gathered from lots of sophisticated imaging strategies, as no method by itself can create total, atomic-level plans of contagious proteins in their natural states. They combined small-angle X-ray scattering (SAXS), X-ray crystallography, and small-angle neutron scattering (SANS) carried out at Berkeley Lab’s Advanced Light Source, Argonne’s Advanced Photon Source, and Oak Ridge’s High Flux Isotope Reactor and Spallation Neutron Source, respectively, on samples of biosynthetically produced RTC.

“Aside from the complexity of the viral system, working during the pandemic was very hard. But we were driven to conduct this research more than anything we have ever done by all the suffering being experienced by families across the country and indeed the world.” – Greg Hura, photographed operating at the ALS beamline utilized for SAXS, in June 2020. Credit: Thor Swift/Berkeley Lab
Despite the amazing obstacles of carrying out science throughout shelter-in-place conditions, the partnership had the ability to work continually for more than 15 months, thanks to moneying for research study and center operations support from the Department of Energy’s Office of Science National Virtual Biotechnology Laboratory (NVBL). During that time, the researchers gathered in-depth information on the RTC’s crucial device proteins and their interactions with RNA. All of their findings were submitted into the open-access Protein Data Bank prior to the journal post’s publication.
Of the lots of structural findings that will aid with drug style, one significant discovery is that assembly of the RTC subunits is exceptionally accurate. Drawing on a mechanical metaphor once again, the researchers compare the assembly procedure to assembling a spring-based device. You can’t put a spring in location when the remainder of the device is currently in position, you need to compress and position the spring at a particular action of assembly or the entire gadget is inefficient. Similarly, the RTC Nsps can’t move into location in any random or disorderly order; they need to follow a particular order of operations.
They likewise determined how among the Nsps particularly acknowledges the RNA particles it acts on, and how it cuts long hairs of copied RNA into their right lengths.
“Having the vaccines is certainly huge. However, why are we satisfied with just this one avenue of defense?” stated Hura. Added Joachimiak: “This was a survey study, and it has identified many directions we and others should pursue very deeply; to tackle this virus we will need multiple ways of blocking its proliferation.”
“Combining information from different structural techniques and computation will be key to achieving this goal,” stated O’Neill.
Due to the resemblance of RTC proteins throughout viral pressures, the group thinks that any drugs established to obstruct RTC activity might work for numerous viral infections in addition to all COVID-19 versions.
Reflecting back to the start of their research study journey, the researchers admire the fortunate timing of all of it. When we began to talk, stated Hura, “we had no idea that this epidemic would soon become a pandemic that would change a generation.”
Reference: “Transient and stabilized complexes of Nsp7, Nsp8, and Nsp12 in SARS-CoV-2 replication” by Mateusz Wilamowski, Michal Hammel, Wellington Leite, Qiu Zhang, Youngchang Kim, Kevin L. Weiss, Robert Jedrzejczak, Daniel J. Rosenberg, Yichong Fan, Jacek Wower, Jan C. Bierma, Altaf H. Sarker, Susan E. Tsutakawa and Sai Venkatesh, 28 June 2021, Biophysical Journal.
DOI: 10.1016/j.bpj.2021.06.006
This research study was supported by the DOE Office of Science through the NVBL, a consortium of DOE nationwide labs concentrated on the action to COVID-19, with financing offered by the Coronavirus CARES Act; and by the National Institutes of Health. The Advanced Light Source, Advanced Photon Source, High Flux Isotope Reactor, and Spallation Neutron Source are DOE Office of Science user centers.





