The synaptic pruning of newborn nerve cells by microglia depends upon phosphatidylserine in the adult brain. This system is necessary for the maturation of newborn nerve cells.
A research study group led by Kazunobu Sawamoto, a teacher at Institute of Brain Science, Nagoya City University Graduate School of Medical Sciences and National Institute for Physiological Sciences, and Chihiro Kurematsu, a fourth-year trainee at Nagoya City University School of Medicine, has actually illuminated the system that manages synaptic pruning of brand-new nerve cells in the adult brain.
In the mammalian brain, neural stem cells exist even after birth, and brand-new nerve cells are produced. As these brand-new nerve cells develop, they form connections called synapses with existing nerve cells to produce practical neural circuits. For the brain to establish and operate usually, it is necessary to preserve a suitable variety of synapses, however the system to manage the variety of synapses has actually not been completely comprehended. Cells called microglia, which exist near afferent neuron, play a crucial function in this procedure. Microglia can “eat” (phagocytose) dead cells and are likewise understood to absorb additional synapses throughout advancement. However, it was unidentified how brand-new nerve cells in the adult brain remove additional synapses throughout maturation.
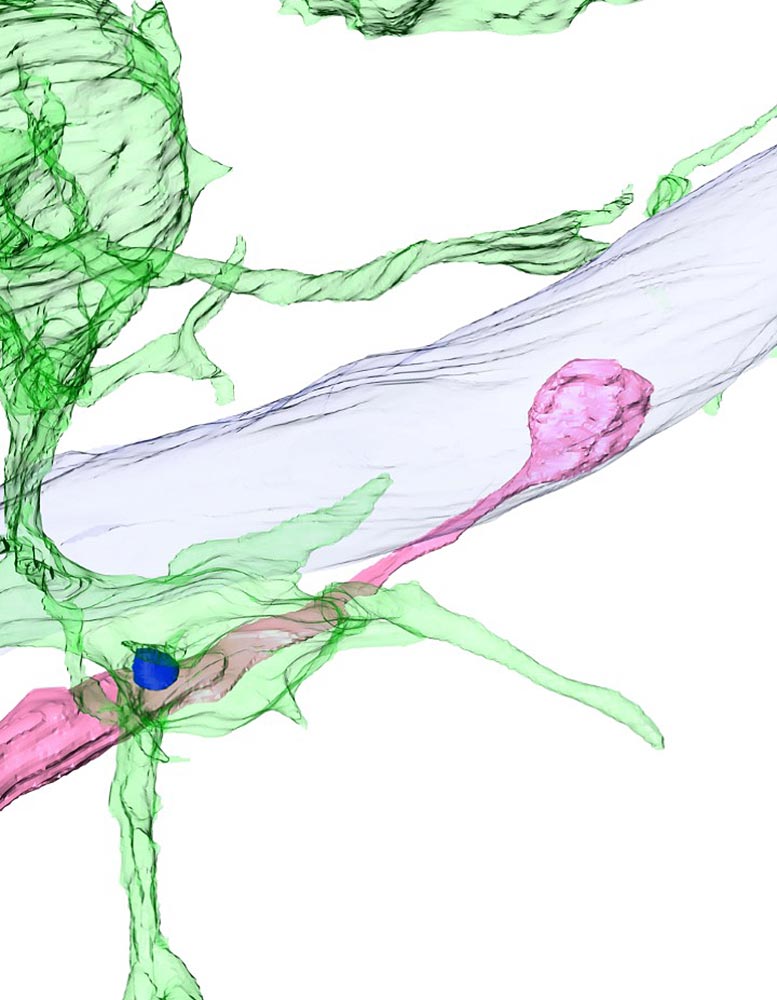
Three- dimensional restoration of electron tiny images revealing a synaptic spinal column (blue) of a brand-new nerve cell (pink), which forms a synapse with another nerve cell (light blue), swallowed up by microglia (green) in the adult mouse olfactory bulb. Credit: © 2022 Kurematsu et al. Originally released in Journal of Experimental Medicine
Sawamoto’s group concentrated on phosphatidylserine (PS), a particle that typically lives inside the cell membrane, however is discovered on the external surface area of dead cells or establishing synapses, where it is acknowledged by microglia. First, the scientists utilized an electron microscopic lense to take a look at the microglia in information and observed that microglia in fact swallow up synapses. Next, they took a look at the localization of PS and discovered that PS is exposed outside the cell membrane at synapses in the adult mouse brain, specifically at less active synapses.
“To study whether microglial PS detection is important for synaptic pruning and normal neuron maturation in the adult brain, we needed to see what happens when PS is masked in living adult mice,” Sawamoto stated.
Finally, they produced genetically customized mice in which PS outside the cell membrane was masked by a protein, and observed microglia and synapses. As an outcome, they discovered that in these mice, microglia might not consume synapses effectively, leading to additional synapses left. Furthermore, nerve cells in these mice revealed electrophysiological irregularities. These results show that the synaptic pruning of newborn nerve cells by microglia is PS-dependent in the adult brain, which this system is necessary for the appropriate maturation of newborn nerve cells.
Recent research studies have actually revealed that brand-new nerve cells are likewise produced in the human brain throughout the neonatal duration, and microglial synaptic pruning is thought to be crucial for postnatal brain advancement.
“We hope that investigating PS-dependent synaptic elimination in mouse models of brain diseases will lead to the development of new therapeutic strategies for human pathological conditions such as autism, where abnormalities in microglia and synaptic density have been observed,” stated Kurematsu.
The complete findings of the research study are released in the Journal of Experimental Medicine
Reference: “Phosphatidylserine-dependent synaptic pruning by microglia in the maturation of adult-born neurons” by Chihiro Kurematsu, Masato Sawada, Masaki Ohmuraya, Motoki Tanaka, Kazuya Kuboyama, Takashi Ogino, Mami Matsumoto, Hisashi Oishi, Hiroyuki Inada, Yuri Ishido, Yukina Sakakibara, Huy Bang Nguyen, Truc Quynh Thai, Shinichi Kohsaka, Nobuhiko Ohno, Maki K. Yamada, Masato Asai, Masahiro Sokabe, Junichi Nabekura, Kenichi Asano, Masato Tanaka and Kazunobu Sawamoto, 17 March 2022, Journal of Experimental Medicine
DOI: 10.1084/ jem.20202304
In addition to Kazunobu Sawamoto and Chihiro Kurematsu, co-authors of this research study short article consist of scientists from Nagoya City University, National Institute for Physiological Sciences, Hyogo College of Medicine, Aichi Developmental Disability Center, University of Medicine and Pharmacy of Ho Chi Minh City, Pham Ngoc Thach University of Medicine, National Institute of Neuroscience, Jichi Medical University, Tokushima Bunri University, Nagoya University, and Tokyo University of Pharmacy and Life Sciences.





