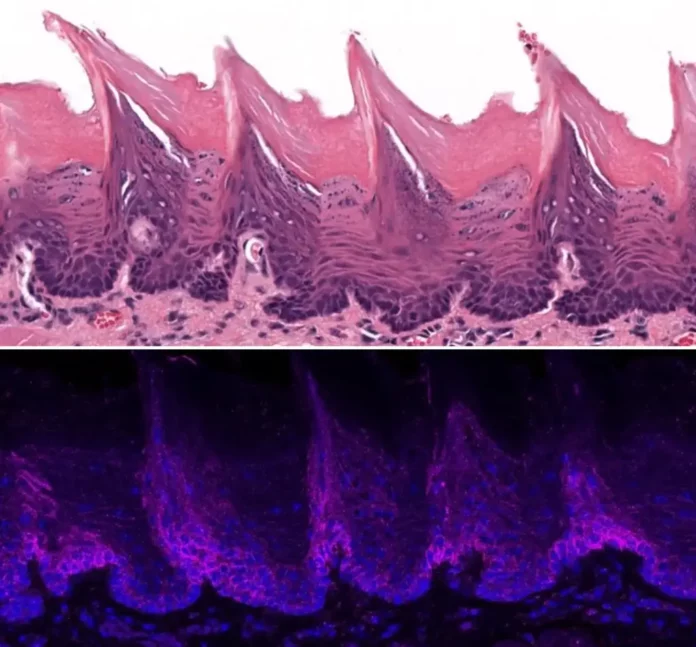
Tissue from a mouse tongue as seen under a microscopic lense (top) and localization of the ORAI1 protein in the tissue on surface area of the mouse tongue (bottom). Credit: Lacruz Lab/ NYU
Protein ORAI1 fuels oral cancers– and might offer an appealing restorative target.
An important protein that functions as a gatekeeper for calcium getting in cells promotes the development of oral cancer and produces discomfort, according to a brand-new research study released on September 5 in the journal Science Signaling led by scientists at NYU College of Dentistry.
Targeting this protein– the ORAI1 calcium channel– might offer a brand-new technique to dealing with oral cancer, which triggers consistent discomfort that gets worse as it advances.
“Our results show that the ORAI1 channel fuels the growth of oral cancer tumors and produces an abundance of molecules that, once secreted, interact with neurons resulting in an increased sensitivity to pain,” stated Ga-Yeon Son, a postdoctoral fellow in the Department of Molecular Pathobiology at NYU College of Dentistry and the research study’s very first author.
The Keepers of the Gates of Heaven
ORAI calcium channels– called after the 3 siblings in Greek folklore who secured evictions of paradise at Mount Olympus– play an essential function in managing just how much calcium gets in cells.
“These calcium channels can be a source of good or bad for cells,” stated Rodrigo Lacruz, teacher of molecular pathobiology at NYU College of Dentistry and the research study’s senior author.
“Calcium entering cells is necessary for many good things, but too much calcium for a long time has the opposite effect.”
Calcium channels have actually been connected to different cancers, particularly cancer development, however couple of research studies have actually taken a look at the function of ORAI1 in cancer and discomfort.
“Calcium influx through ORAI1 channels has been well known to contribute to the regulation of gene expression by activating gene transcription factors in the cells. Notably, our investigation extends its function in regulating gene expression to altering oral cancer pain,” stated Son.
Less ORAI1, Less Cancer Growth and Pain
The scientists very first evaluated tissue samples from human oral cancer growths and healthy tongues. They discovered that the ORAI1 gene, which includes directions for producing the ORAI1 calcium channel, was greatly overexpressed in the growths however not in healthy tissue.
They then took a look at human oral cancer cells and discovered that triggering the ORAI1 calcium channel (however not other calcium channels) triggered a big increase of calcium into cancer cells. This increase led to the boost of a calcium-dependent enzyme called matrix metalloprotease 1 (MMP1) that is produced beyond cancer cells. MMP1 is plentiful in several kinds of cancer, consisting of oral cancer, where its overexpression is connected with transition and bad diagnosis.
Removing the ORAI1 gene from oral cancer cells altered the course of the illness in animal research studies. When mice were inoculated with cancer cells doing not have the ORAI1 gene, growths grew more gradually and were less agonizing.
“These findings demonstrate an important role for ORAI1 in oral cancer progression and pain, but what is the mechanism? We wondered if MMP1 could be the messenger relaying pain,” stated Lacruz.
In partnership with NYU Pain Research Center researchers Rajesh Khanna and Yi Ye, the group took a look at the levels of MMP1 revealed in the fluid surrounding oral cancer cells and saw that cells doing not have the ORAI1 gene produced less MMP1 into the surrounding fluid. They integrated the fluid with nerve cells from the trigeminal ganglia, a collection of nerves in the face that send discomfort in oral cancer. The fluid from cancer cells without the ORAI1 gene did not generate a strong reaction from the nerve cells, however the MMP1-rich fluid from cells with ORAI1 stimulated a boost in action capacities, the essential signal for discomfort transmission.
“This gives us evidence that an abundance of MMP1 may generate increased sensitivity to pain,” stated Lacruz.
The scientists likewise ran try outs unusual however non-cancerous cells. When they overexpressed the ORAI1 gene in these non-invasive cells, they ended up being intrusive, raising the possibility that ORAI1 might contribute in cells changing from non-cancerous to malignant cells.
Potential Treatment Pathways
Several FDA-approved drugs obstruct the ORAI1 calcium channel, however they have actually not yet been evaluated in oral cancer. In future research studies, the scientists will see whether nanoparticles can be packed with an ORAI-blocking drug and specifically provided into the tongues of animal designs to stop oral cancer development and discomfort.
“In light of the ongoing opioid crisis, our study paves the way for validating novel pain treatments in oral cancer,” stated Rajesh Khanna, director of the NYU Pain Research Center, teacher of molecular pathobiology at NYU Dentistry, and a co-author of the research study.
“Ultimately, our hope is that targeting the ORAI1 channel in oral cancer can prevent or delay the progression from oral epithelial dysplasia to oral cancer tumors and concurrently alleviate the pain burden experienced by oral cancer patients,” included Son.
Reference: “The Ca 2+ channel ORAI1 is a regulator of oral cancer development and nociceptive discomfort” by Ga-Yeon Son, Nguyen Huu Tu, Maria Daniela Santi, Santiago Loya Lopez, Guilherme H. Souza Bomfim, Manikandan Vinu, Fang Zhou, Ariya Chaloemtoem, Rama Alhariri, Youssef Idaghdour, Rajesh Khanna, Yi Ye and Rodrigo S. Lacruz, 5 September 2023, Science Signaling
DOI: 10.1126/ scisignal.adf9535
Additional research study authors consist of Nguyen Huu Tu, Maria Daniela Santi, Santiago Loya Lopez, and Guilherme H. Souza Bomfim of NYU College of Dentistry; Manikandan Vinu, Ariya Chaloemtoem, Rama Alhariri, and Youssef Idaghdour of NYU Abu Dhabi; and Fang Zhou of NYU Langone Health.
The research study was supported by the National Institute of Dental and Craniofacial Research (DE027981, DE027679, R01 DE029493), < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>National Institutes of Health</div><div class=glossaryItemBody>The National Institutes of Health (NIH) is the primary agency of the United States government responsible for biomedical and public health research. Founded in 1887, it is a part of the U.S. Department of Health and Human Services. The NIH conducts its own scientific research through its Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program. With 27 different institutes and centers under its umbrella, the NIH covers a broad spectrum of health-related research, including specific diseases, population health, clinical research, and fundamental biological processes. Its mission is to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" >NationalInstitutes of Health HEALInitiative( R01 DE032501),NationalInstitute ofNeurologicalDisorders andStroke( NS098772, NS120663), andNationalInstitute onDrugAbuse (DA042852).