Researchers have actually presented an ingenious approach for producing heterocyclic substances utilizing TiO2 and noticeable light, using a more sustainable and economical method compared to standard high-energy procedures. Credit: SciTechDaily.com
Researchers propose a brand-new titanium dioxide-catalyzed method for manufacturing thiochromenopyrroledione derivatives in blue light.
Heterocyclic substances are natural particles with a ring structure making up a minimum of 2 or more components. In most cases, these rings are made up of carbon atoms together with several other components such as nitrogen, oxygen, or sulfur. They are extremely desired as basic materials in the chemical and pharmaceutical market, owing to their adaptability and outstanding physiological activities.
While a number of techniques are offered for manufacturing these substances, the majority of them include heat and pressure conditions, or using rare-earth element drivers, contributing to the financial and ecological expense of producing heterocyclic natural substances.
Innovative Synthesis Method
Now, nevertheless, a group of scientists from Japan and Bangladesh have actually proposed a basic yet reliable approach for getting rid of these difficulties. Their research study was just recently released in the journal Advanced Synthesis and Catalysis Using the proposed method, the group showed the synthesis of 20 sulfur-containing heterocyclic substances in the existence of photocatalyst titanium dioxide (TiO 2) and noticeable light.
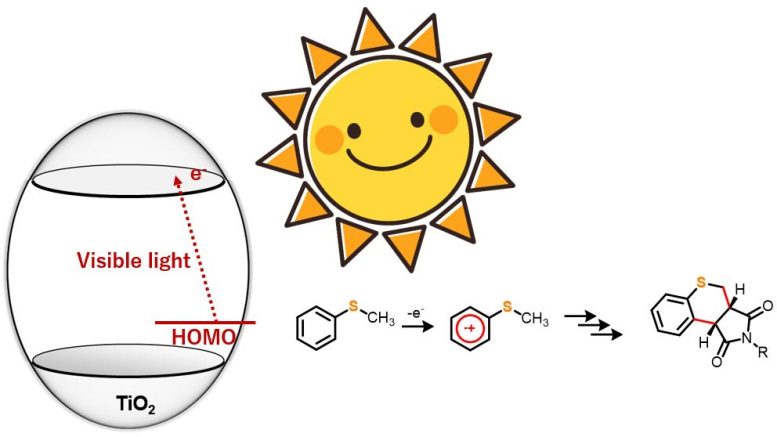
A group of scientists now provides an environmentally friendly and ingenious method for the blue light-promoted synthesis of heterocyclic thiochromenopyrroledione derivatives catalyzed by titanium dioxide. Credit: Professor Yutaka Hitomi from Doshisha University
The research study was led by Professor Yutaka Hitomi from the Department of Applied Chemistry, Graduate School of Science and Engineering, Doshisha University, and co-authored by aPh D. prospect Pijush Kanti Roy from Doshisha University, Associate Professor Sayuri Okunaka from Tokyo City University, andDr Hiromasa Tokudome from Research Institute, TOTO Ltd.
The Role of TiO 2 in Organic Synthesis
TiO 2 as a photocatalyst for driving natural responses has actually caught the attention of artificial chemists for a while now. However, numerous such procedures need ultraviolet light to set off the response. In this research study, nevertheless, the research study group discovered that under anaerobic conditions, sulfur-containing natural substances like thioanisole derivatives, when struck with blue light, responded with maleimide derivatives to form double carbon– carbon bonds, yielding a brand-new heterocyclic natural substance.
“We observed that while ultraviolet light generates highly oxidative holes, our approach allows for the selective one-electron oxidation of the substrate molecules using visible light. This approach can thus be employed in various organic chemical reactions,” discussesProf Hitomi.
Experimental Procedures and Findings
The scientists picked 5 4-substituted thioanisoles and 4 N-substituted maleimides for the annulation or ring development responses. The group irradiated the beginning product with blue light (wavelength > > 420 nm) however observed no response. However, presenting TiO 2 into the response system resulted in the synthesis of 20 various thiochromenopyrroledione derivatives with moderate-to-high yield.
They discovered that within 12 hours of direct exposure to blue light, the response in between thioanisole and N-benzylmaleimide resulted in the development of a thiochromenopyrroledione derivative with 43% yield, which was close to the theoretical optimum yield of 50%.
The research study group likewise observed substituent impacts in the responses to comprehend the matching mechanistic elements. From the outcomes, they postulated that the response continues through charge transfer from thioanisole to the conduction band of TiO 2 Furthermore, they recommended that irradiation with blue light set off one-electron oxidation of thioanisole, which even more started the generation of a-thioalkyl radicals through deprotonation.
Potential and Impact of the Research
In summary, this brand-new and refined method shows the capacity of TiO 2 for noticeable light photocatalysis for natural synthesis. It likewise supplied important insights into the chemistry of complicated heterocyclic substance synthesis. Going ahead, this method can open brand-new possibilities for transitioning from present resource-intensive commercial chemical procedures to a more energy-efficient system.
Highlighting the significance and ramifications of this research study,Prof Hitomi states, “What drove our study was the desire to aid in the development of a sustainable chemical industry, and our findings appear to be a positive step in this direction.”
Adding even more, he states, “We believe that the widespread adoption of this visible light-driven technology could assist in accessible and affordable synthesis of pharmaceuticals, with its profound impacts on the health and well-being of millions of people worldwide.”
Thanks to the efforts ofProf Hitomi and his group, their research study has actually opened brand-new opportunities in the field of natural synthesis, with the possible to change several chemical markets.
Reference: “Blue Light-Promoted Synthesis of Thiochromenopyrroledione Derivatives via Titanium Dioxide-Catalyzed Dual Carbon–Carbon Bond Formation with Thioanisole and Maleimide Derivatives” by Pijush Kanti Roy, Sayuri Okunaka, Hiromasa Tokudome and Yutaka Hitomi, 15 November 2023, Advanced Synthesis & & Catalysis
DOI: 10.1002/ adsc.202301021
This work was supported by the PRESTO Grant Number JPMJPR17 S8 (YH) from Japan Science and Technology (JST) and JSPS KAKENHI Grant Number JP22 K05360 (YH).
About Professor Yutaka Hitomi from Doshisha University, Japan
Yutaka Hitomi is a Professor at the Department of Applied Chemistry, Graduate School of Science and Engineering, Doshisha University,Japan Prof. Hitomi got his Master’s and Doctorate in Engineering from the Kyoto University,Japan In 2016, he got the Nagase Research Promotion Award for his contributions to nanotechnology, biochemistry, and artificial chemistry. The present research study locations ofProf Hitomi and his group consist of oxidation responses, bioinorganic, catalytic, and coordination chemistry. He has actually released 95 publications up until now, which have actually gotten more than 2,300 citations.





