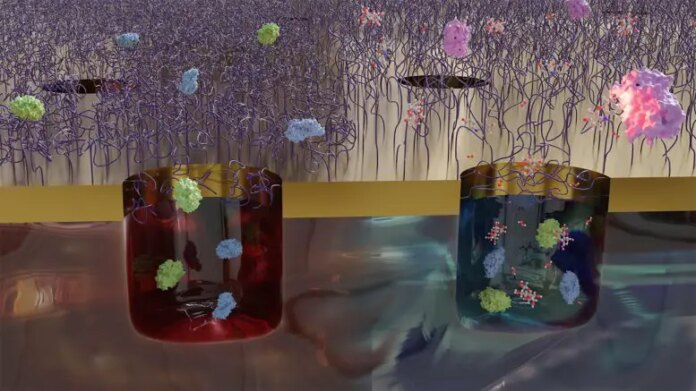
The image reveals the protein traps, which include nanoscale chambers and polymers that form gates above. These “doors” are opened up by increasing the temperature level by about 10 degrees, which is done electrically. Then the polymers alter their shape to a more compact state so that proteins can pass in and out. Credit: Chalmers University of Technology|Julia Järlebark
Protein clumps are linked in a number of tough illness such as ALS, < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>Alzheimer’s</div><div class=glossaryItemBody>Alzheimer's disease is a disease that attacks the brain, causing a decline in mental ability that worsens over time. It is the most common form of dementia and accounts for 60 to 80 percent of dementia cases. There is no current cure for Alzheimer's disease, but there are medications that can help ease the symptoms.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" >Alzheimer’s, andParkinson’s.Understanding the interaction of these proteins is complex.However, scientists atChalmersUniversity ofTechnology, (************************************************************************************************************************************* )have actually found a brand-new technique for recording numerous proteins in nano-sized traps.This unique technique permits extraordinary insights into protein habits.
“We believe that our method has great potential to increase the understanding of early and dangerous processes in a number of different diseases and eventually lead to knowledge about how drugs can counteract them,” statesAndreasDahlin, teacher atChalmers, who led the research study job.
Proteins that form clumps in our bodies trigger a a great deal of illness, consisting of ALS,Alzheimer’s, and(**************************************************************************************************************************************************** )’s. A much better understanding of how the clumps form might cause reliable methods to liquify them at an early phase, or perhaps avoid them from forming entirely.

AndreasDahlin,Professor,Department ofChemistry andChemicalEngineering atChalmersUniversity ofTechnologyCredit:ChalmersUniversity ofTechnology|Mikael Terfors
Today, there are different methods for studying the later phases of the procedure, when the clumps have actually ended up being big and formed long chains, however previously it has actually been tough to follow the early advancement, when they are still extremely little. These brand-new traps can now assist to fix this issue.
Can research study greater concentrations for a longer time
The scientists explain their work as the world’s tiniest gates that can be opened and closed at the touch of a button. The gates end up being traps, that lock the proteins inside chambers at the < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>nanoscale</div><div class=glossaryItemBody>The nanoscale refers to a length scale that is extremely small, typically on the order of nanometers (nm), which is one billionth of a meter. At this scale, materials and systems exhibit unique properties and behaviors that are different from those observed at larger length scales. The prefix "nano-" is derived from the Greek word "nanos," which means "dwarf" or "very small." Nanoscale phenomena are relevant to many fields, including materials science, chemistry, biology, and physics.</div>" data-gt-translate-attributes="(** )" > nanoscale(*************** ). The proteins are avoided from leaving, extending the time they can be observed at this level from one millisecond to a minimum of one hour.The brand-new technique likewise makes it possible to confine a number of hundred proteins in a little volume, an essential function for additional understanding.
“The clumps that we want to see and understand better consist of hundreds of proteins, so if we are to study them, we need to be able to trap such large quantities. The high concentration in the small volume means that the proteins naturally bump into each other, which is a major advantage of our new method,” statesAndreasDahlin
In order for the strategy to be utilized to study the course of particular illness, continued advancement of the technique is needed.
“The traps need to be adapted to attract the proteins that are linked to the particular disease you are interested in. What we’re working on now is planning which proteins are most suitable to study,” states Andreas Dahlin.
How the brand-new traps work
The gates that the scientists have actually established include so-called polymer brushes placed at the mouth of nano-sized chambers. The proteins to be studied are included in a liquid service and are drawn in to the walls of the chambers after an unique chemical treatment.
When evictions are closed, the proteins can be devoid of the walls and begin moving towards each other. In the traps, you can study specific clumps of proteins, which supplies far more info compared to studying numerous clumps at the very same time. For example, the clumps can be formed by various systems, have various sizes and various structures.
Such distinctions can just be observed if one analyses them one by one. In practice, the proteins can be maintained in the traps for nearly any length of time, however at present, the time is restricted by the length of time the chemical marker– which they need to be supplied with to end up being noticeable– stays. In the research study, the scientists handled to keep presence for as much as an hour.
Reference: “Stable trapping of multiple proteins at physiological conditions using nanoscale chambers with macromolecular gates” by Justas Svirelis, Zeynep Adali, Gustav Emilsson, Jesper Medin, John Andersson, Radhika Vattikunta, Mats Hulander, Julia Järlebark, Krzysztof Kolman, Oliver Olsson, Yusuke Sakiyama, Roderick Y. H. Lim and Andreas Dahlin, 23 August 2023, < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>Nature Communications</div><div class=glossaryItemBody><em>Nature Communications</em> is a peer-reviewed, open-access, multidisciplinary, scientific journal published by Nature Portfolio. It covers the natural sciences, including physics, biology, chemistry, medicine, and earth sciences. It began publishing in 2010 and has editorial offices in London, Berlin, New York City, and Shanghai. </div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" >NatureCommunications
DOI:101038/ s41467 -023-40889 -4
The research study was moneyed by theEuropeanResearchCouncil,Erling-PerssonFamilyFoundation