Researchers at Hokkaido University have actually developed particles efficient in turning internally when exposed to light, possibly transforming molecular switches, computing, and drug advancement. Inspired by natural procedures, this advancement uses brand-new opportunities for exact molecular control and applications in targeted treatments and bioactive systems.
Molecules that are caused by light to turn large groups around main bonds when exposed to light might result in the development of light-activated bioactive systems, molecular switches, to name a few applications.
Researchers at Hokkaido University, led by Assistant Professor Akira Katsuyama and Professor Satoshi Ichikawa at the Faculty of Pharmaceutical Sciences, have actually extended the toolkit of artificial chemistry by making a brand-new classification of particles that can be caused to go through an internal rotation on interaction with light.
Similar procedures are thought to be essential in some natural biological systems. Synthetic variations may be made use of to carry out photochemical changing functions in molecular computing and picking up innovations, or in bioactive particles consisting of drugs. They report their findings in Nature Chemistry
Advancements in Photochemistry
“Achieving a system like ours has been a significant challenge in photochemistry,” statesKatsuyama “The work makes an important contribution to an emerging field in molecular manipulation.”
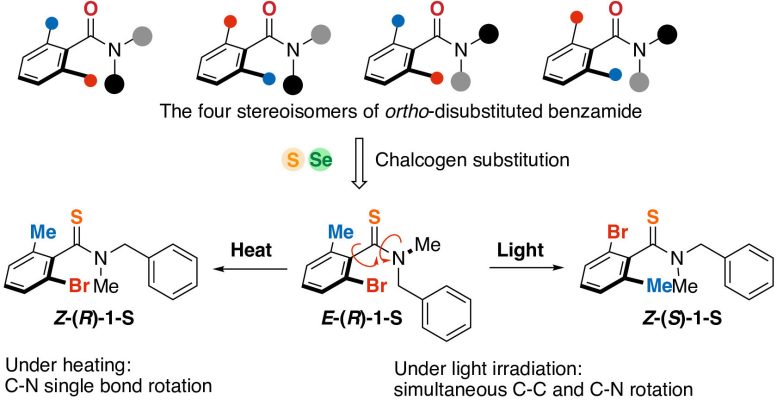
In this research study, sulfur or selenium atoms were presented into a benzamide derivative (top). The resulting particles went through isomerization when exposed to light or heat. Credit: Shotaro Nagami, et al. NatureChemistry February 28, 2024

The particles manufactured in this research study form various isomers when irradiated with blue light. Credit: Akira Katsuyama
Insights into the possibilities for light to substantially change molecular conformations have actually originated from analyzing some natural proteins. These consist of the rhodopsin particles in the retina of the eye, which play an important function in transforming light into the electrical signals that develop our sense of vision in the brain. Details are emerging of how the absorption of light energy can cause a twisting rearrangement of part of the rhodopsin particle, needed for it to perform its biological function.
“Mimicking this in synthetic systems might create molecular-level switches with a variety of potential applications,” Katsuyama describes.
Innovations in Molecular Rotation
An essential development by the Hokkaido group was to accomplish photo-induced ( i.e., light-driven) rotation of molecular groups around a series of chemical bonds that include a nitrogen < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>atom</div><div class=glossaryItemBody>An atom is the smallest component of an element. It is made up of protons and neutrons within the nucleus, and electrons circling the nucleus.</div>" data-gt-translate-attributes ="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex =(****************************************************************** )function ="link" > atom (************************ )together with other bonded carbon atoms.
(************ )The rotational homes were allowed by including molecular parts which contained an atom from the‘chalcogen’ group of aspects in the table of elements, particularly sulfur or selenium, to an easy natural particle: an amide substance.This brought a brand-new level of control and adaptability to artificial photo-induced rotational systems.

In this research study, sulfur or selenium atoms were presented into a benzamide derivative( top).The resulting particles went through isomerization when exposed to light or heat.Credit:ShotaroNagami, et al.NatureChemistryFebruary28,2024
Some of the chemical groups that turn around the main bonds were fairly big, based upon rings of 6 bonded carbon atoms. This assisted in the massive molecular modifications that may be needed for useful usage in molecular changing systems.
In addition to showing the photo-induced modifications, the group likewise carried out theoretical estimations that provided insights into the most likely systems by which the rearrangements continued. The group likewise checked out the results of temperature level on the improvements. The mix of theoretical and speculative work need to assist assist future research study towards checking out and managing adjustments to the systems currently attained.
“Our next research priority is focused on the potential of our methods for making new bioactive molecules activated by light. These could be applied in biological research or possibly developed as drugs,” Ichikawa concludes.
Using light to trigger the conformational modifications enables control over where and when the modifications take place. This might be important for specifically targeted applications in biological systems, consisting of ultimate healing possibilities.
Reference: “Photoinduced dual bond rotation of a nitrogen-containing system realized by chalcogen substitution” by Shotaro Nagami, Rintaro Kaguchi, Taichi Akahane, Yu Harabuchi, Tohru Taniguchi, Kenji Monde, Satoshi Maeda, Satoshi Ichikawa and Akira Katsuyama, 28 February 2024, Nature Chemistry
DOI: 10.1038/ s41557-024-01461 -9
The research study was moneyed by the Japan Society for the Promotion of Science, the Japan Agency for Medical Research and Development, the Japan Science and Technology Agency, and the Akiyama Life Science Foundation.





