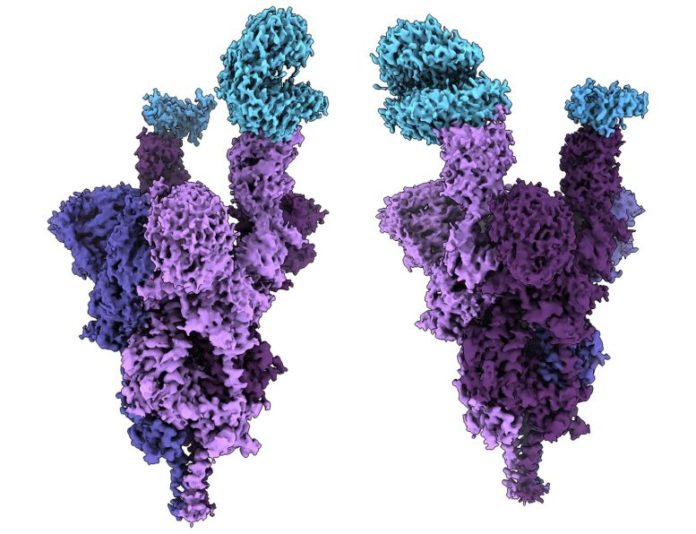
Atomic structure of the Omicron alternative spike protein (purple) bound with the human ACE2 receptor (blue). Credit: UBC Faculty of Medicine
Findings clarified elements behind Omicron’s increased transmissibility, consisting of strong antibody evasion and binding with human cells.
Researchers at UBC’s professors of medication have actually carried out the world’s very first molecular-level structural analysis of the Omicron alternative spike protein. The findings were released on January 20, 2022, in Science
The analysis– done at near atomic resolution utilizing cryo-electron microscopy– exposes how the greatly altered Omicron alternative connects to and contaminates human cells.
“Understanding the molecular structure of the viral spike protein is important as it will allow us to develop more effective treatments against Omicron and related variants in the future,” stated lead authorDr Sriram Subramaniam (he/him), teacher in UBC’s department of biochemistry and molecular biology. “By analyzing the mechanisms by which the virus infects human cells, we can develop better treatments that disrupt that process and neutralize the virus.”
The spike protein, which lies on the exterior of a coronavirus, allows SARS-CoV-2 to get in human cells. The Omicron version has an unmatched 37 anomalies on its spike protein– 3 to 5 times more than previous versions.
The structural analysis exposed that numerous anomalies (R493, S496, and R498) develop brand-new salt bridges and hydrogen bonds in between the spike protein and the human cell receptor called ACE2. The scientists concluded that these brand-new bonds appear to increase binding affinity– how highly the infection connects to human cells– while other anomalies (K417 N) reduce the strength of this bond.
“Overall, the findings show that Omicron has greater binding affinity than the original virus, with levels more comparable to what we see with the Delta variant,” statedDr Subramaniam. “It is remarkable that the Omicron variant evolved to retain its ability to bind with human cells despite such extensive mutations.”
The scientists carried out even more experiments revealing that the Omicron spike protein shows increased antibody evasion. In contrast to previous versions, Omicron revealed quantifiable evasion from all 6 monoclonal antibodies evaluated, with total escape from 5. The version likewise showed increased evasion of antibodies gathered from immunized people and unvaccinated COVID-19 clients.
“Notably, Omicron was less evasive of the immunity created by vaccines, compared to immunity from natural infection in unvaccinated patients. This suggests that vaccination remains our best defense,” statedDr Subramaniam.
Based on the observed boost in binding affinity and antibody evasion, the scientists state that the spike protein anomalies are most likely contributing elements to the increased transmissibility of the Omicron version.
Next,Dr Subramaniam states his research study group will utilize this understanding to support the advancement of more reliable treatments.
“An important focus for our team is to better understand the binding of neutralizing antibodies and treatments that will be effective across the entire range of variants, and how those can be used to develop variant-resistant treatments.”
Reference: “SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein–ACE2 complex” by Dhiraj Mannar, James W. Saville, Xing Zhu, Shanti S. Srivastava, Alison M. Berezuk, Katharine S. Tuttle, Ana Citlali Marquez, Inna Sekirov and Sriram Subramaniam, 20 January 2022, Science
DOI: 10.1126/ science.abn7760