Research reveals deficiency of mitochondria in nerve cell axons results in protein accumulation, an essential consider neurodegenerative illness, providing a brand-new target for treatment. Credit: SciTechDaily.com
Key path recognized for how deficiency of axonal mitochondria interrupts autophagy.
Researchers from Tokyo Metropolitan University have actually recognized how proteins gather unusually in nerve cells, a function of neurodegenerative illness like < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>Alzheimer’s</div><div class=glossaryItemBody>Alzheimer's disease is a disease that attacks the brain, causing a decline in mental ability that worsens over time. It is the most common form of dementia and accounts for 60 to 80 percent of dementia cases. There is no current cure for Alzheimer's disease, but there are medications that can help ease the symptoms.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" >Alzheimer’s .They utilized fruit flies to reveal that deficiency of mitochondria in axons can straight result in protein build-up.At the exact same time, substantially high quantities of a protein called eIF2β were discovered.Restoring the levels to typical resulted in a healing in protein recycling.Such findings guarantee brand-new treatments for neurodegenerative illness.
TheCellularFactory:ProteinProduction andDisassembly
Every cell in our bodies is a hectic factory, where proteins are continuously being produced and taken apart.(********************************************************************************************************************************************************************** )modifications or lapses in either the production or recycling stages can result in severe health problems.Neurodegenerative illness such as(************************************************************************************************************************************************************************** )’s andAmyotrophic(************************************************************************************************************************** )Sclerosis( ALS ), for instance, are understood to be accompanied by an irregular accumulation of proteins in nerve cells. However, the trigger behind this build-up stays unidentified.
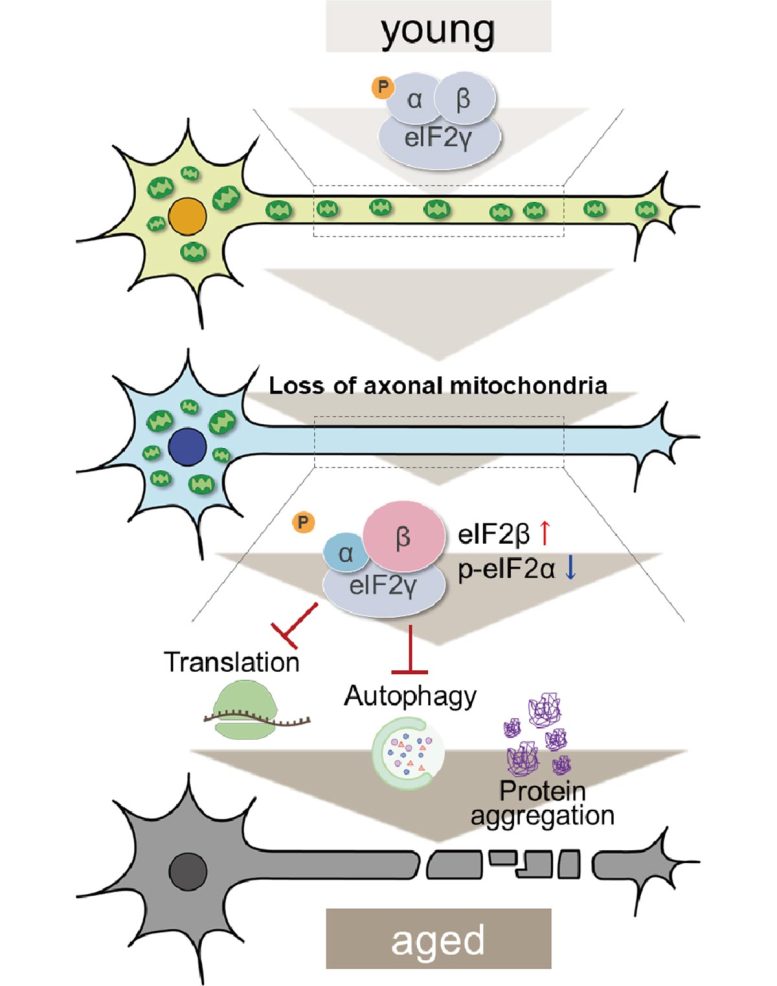
As axonal mitochondria are lost, modifications in the subunits of eIF2 are seen which obstruct translation and autophagy and trigger the build-up of proteins in cells. Credit: Tokyo Metropolitan University
Research Using Drosophila Fruit Flies
A group led by Associate Professor Kanae Ando of Tokyo Metropolitan University has actually been attempting to identify the reasons for unusual protein accumulation by studying Drosophila fruit flies, a typically studied design organism that has numerous crucial resemblances with human physiology.
They concentrated on the existence of mitochondria in axons, the long tendril-like appendages that extend of nerve cells and form the needed connections that permit signals to be sent inside our brains. It is understood that the levels of mitochondria in axons can drop with age, and throughout the development of neurodegenerative illness.
Findings on Mitochondria Depletion and Protein Accumulation
Now, the group has actually found that the deficiency of mitochondria in axons has a direct bearing on protein accumulation. They pre-owned genetic engineering to reduce the production of milton, an essential protein in the transportation of mitochondria along axons.
It was discovered that this resulted in unusual levels of protein developing in fruit fly nerve cells, an outcome of the breakdown of autophagy, the recycling of proteins in cells.
Through proteomic analysis, they had the ability to recognize a considerable upregulation in eIF2β, an essential subunit of the eIF2 protein complex accountable for the initiation of protein production (or translation). The eIF2α subunit was likewise discovered to be chemically customized. Both of these concerns obstruct the healthy action of eIF2.
Implications for Neurodegenerative Disease Treatment
Importantly, by synthetically reducing levels of eIF2β, the group found that they might bring back the autophagy that was lost and restore a few of the nerve cell function that suffered due to axonal mitochondria loss. This not just reveals that deficiency of mitochondria in axons can trigger unusual protein build-up, however that this occurs through upregulation of eIF2β.
As populations age and the occurrence of neurodegenerative conditions continues to increase, the group’s findings provide a crucial action in establishing treatments to fight these severe health problems.
Reference: “Axonal distribution of mitochondria maintains neuronal autophagy during aging via eIF2β” by Kanako Shinno, Yuri Miura, Koichi M. Iijima, Emiko Suzuki and Kanae Ando, 25 March 2024, eLife
DOI: 10.7554/ eLife.955761
This work was supported by a Sasakawa Scientific Research Grant (2021-4087), the Takeda Science Foundation, a Hoansha Foundation Grant, a research study award from the Japan Foundation for Aging and Health and the Novartis Foundation (Japan) for the Promotion of Science, a Grant- in-Aid for Scientific Research on Challenging Research (Exploratory) [JSPS KAKENHI Grant Number 19K21593], NIG-JOINT (National Institute of Genetics, 71 A2018, 25 A2019), and the TMU Strategic Research Fund for Social Engagement.





