Discoveries about the end-replication issue show both telomerase and the CST–Pol α-primase complex are vital for chromosome defense, recommending a modification in the science of telomeres and prospective influence on congenital diseases. Credit: SciTechDaily.com
Recent research study challenges the enduring understanding of the end-replication issue in < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>DNA</div><div class=glossaryItemBody>DNA, or deoxyribonucleic acid, is a molecule composed of two long strands of nucleotides that coil around each other to form a double helix. It is the hereditary material in humans and almost all other organisms that carries genetic instructions for development, functioning, growth, and reproduction. Nearly every cell in a person’s body has the same DNA. Most DNA is located in the cell nucleus (where it is called nuclear DNA), but a small amount of DNA can also be found in the mitochondria (where it is called mitochondrial DNA or mtDNA).</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" > DNA, exposing 2 unique problems instead of one.
Half a century earlier, researchersJimWatson andAlexey(********************************************************************************************************************* )individually understood that there was an issue with how our DNA gets copied. A peculiarity of direct DNA duplication determined that telomeres that secure completions of chromosomes need to have been growing much shorter with each round of duplication, a phenomenon called the end-replication issue.
Telomerase: ASolutionEmerges
But an option was upcoming:LizBlackburn andCarolGreider found telomerase, an enzyme that includes the telomeric repeats to the ends of chromosomes. “Case closed, everybody thought,” states Rockefeller’s Titia de Lange.
Now, brand-new research study released in Nature recommends that there are 2 end-replication issues, not one. Further, telomerase is just part of the service– cells likewise utilize the CST–Pol α-primase complex, which has actually been thoroughly studied in de Lange’s lab. “For many decades we thought we knew what the end-replication problem was and how it was solved by telomerase,” states deLange “It turns out we had missed half the problem.”
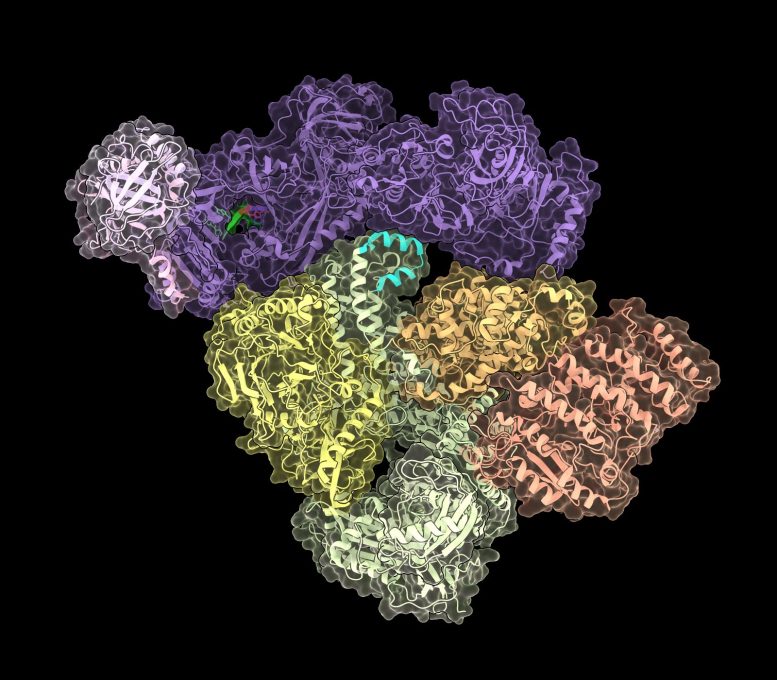
CST–Pol α/ primase, the enzyme that fixes the recently found end-replication issue. Credit: Sarah Cai
The Leading-Strand Problem
Since the description of the DNA double helix, it is understood that DNA has 2 complementary hairs running in opposite instructions– one from 5 ′ to 3 ′; the other from 3 ′ to 5 ′. When DNA is duplicated, the 2 hairs are separated by the duplication equipment, likewise called the replisome. The replisome copies the 3 ′ to 5 ′ hair without disturbance, a procedure described as leading-strand synthesis. But the other hair is manufactured in other words backwards actions from numerous pieces (Okazaki pieces) that are later on sewn together.
The procedure is relatively direct till completions of the chromosomes. When copying the telomere, leading-strand DNA duplication need to copy the CCCTAA repeats to produce the TTAGGG repeat hair, while lagging-strand synthesis ought to do the opposite, making brand-new CCCTAA repeats. The end-replication issue develops since leading hair synthesis stops working to replicate the tail end of the telomere, leaving a blunt leading-end telomere without it particular and vital 3′ overhang. Telomerase fixes this issue by including single-stranded TTAGGG repeats to the telomere end. As for the lagging-strand, DNA synthesis need to not have an issue. It might begin the last Okazaki piece someplace along the 3′ overhang.
“The DNA replication machinery cannot fully duplicate the end of a linear DNA, much the same way that you can’t paint the floor under your feet,” states Hiro Takai, senior personnel researcher in the de Lange laboratory and lead author on the paper.
CST–Pol α/ primase, the enzyme that fixes the recently found end-replication issue. Credit: Sarah Cai
The Lagging-Strand Problem
As descriptions of biological procedures go, this design looked water tight. Until Takai made an unexpected discovery while dealing with cells that did not have molecular equipment called the CST–Pol α-primase complex. He and others had actually formerly revealed that CST–Pol α-primase can renew CCCTAA repeats at telomeres that had actually been assaulted by DNA-degrading enzymes called nucleases. This brand-new information exposed something unforeseen: not just was the leading hair in requirement of aid– he discovered proof that completion of the delayed hair might likewise not be manufactured by the replisome.
Takai’s work recommended that the end-replication issue was two times as severe as formerly believed, affecting both hairs of DNA. “The results just didn’t fit with the model for telomere replication,” de Lange states. “At that point, Hiro and I realized that either his results were not right or the model was wrong. As his results seemed very solid to me, we needed to revisit the model.”
De Lange called Joseph T. P. Yeeles, a biochemist who studies DNA duplication at the Laboratory of Molecular Biology in Cambridge (the very same laboratory where Watson and Crick dealt with the structure of the DNA double helix). Yeeles concurred that it would be excellent to take a close take a look at how the replisome acts at the end of a direct DNA design template. Could the replisome usage a 3′ overhang to make the last Okazaki piece, as was proposed?
The outcomes of Yeeles’ in vitro duplication experiments were really clear. The replisome does not produce Okazaki pieces on the 3′ overhang; it really stops lagging-strand synthesis long before the leading hair reaches the 5′ end. This 2nd end-replication issue indicates that both hairs of DNA will reduce with each department. Telomerase was just avoiding this from occurring at the leading hair and Hiro’s information recommended that CST–Pol α-primase repaired the 2nd end-replication issue, that of the delayed hair.
Takai invested the next 4 years creating brand-new assays to verify Yeeles’ findings in vivo. He had the ability to determine just how much DNA is lost due to the lagging-strand end-replication issue, exposing the number of CCCAAT repeats require to be included by CST–Pol α-primase to keep telomeres undamaged.
Implications and Future Directions
The results modification our understanding of telomere biology– needing modification of the books. But the findings might likewise have medical ramifications. Individuals who acquire anomalies in CST–Pol α-primase struggle with telomere conditions, such as Coats plus syndrome, which is identified by an eye condition and irregularities in the brain, bones, and GI system. Through a much better understanding of how we preserve our telomeres, strides might one day be made in attending to these ravaging conditions.
Reference: “Cryo-EM structure of the human CST–Polα/primase complex in a recruitment state” by Sarah W. Cai, John C. Zinder, Vladimir Svetlov, Martin W. Bush, Evgeny Nudler, Thomas Walz and Titia de Lange, 16 May 2022, < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>Nature Structural & Molecular Biology</div><div class=glossaryItemBody><em>Nature Structural & Molecular Biology</em> is a scientific journal that publishes research articles in the fields of structural biology and molecular biology. Structural biology is the study of the three-dimensional structures of biological molecules, including proteins, nucleic acids, and carbohydrates, and how they function in cells. Molecular biology is the study of the processes that occur within cells at the molecular level, including the regulation of gene expression and the structure and function of cellular components such as enzymes and membranes.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" & function ="link" >NatureStructural &MolecularBiology
DOI:101038/ s41594-022-00766- y





