A research study by CUNY ASRC scientists, utilizing X-ray crystallography under various conditions, exposed numerous shapes of a disease-related protein, using brand-new opportunities for drug advancement. Credit: SciTechDaily.com
New crystallography experiments utilizing high pressure and heat to expose how proteins alter shape might advance the advancement of unique drugs.
Proteins do the heavy lifting of carrying out biochemical functions in our bodies by binding to metabolites or other proteins to finish jobs. To do this effectively, protein particles frequently shape-shift to permit particular binding interactions that are required to carry out complex, accurate chemical procedures.
Research on Protein Structures
A much better understanding of the shapes proteins handle would provide scientists essential insight into stopping or dealing with illness, however present approaches for exposing these vibrant, three-dimensional types use researchers restricted info.
To address this understanding space, a group from the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC) developed an experiment to check whether carrying out X-ray crystallography imaging utilizing raised temperature level versus raised pressure would expose unique shapes. The outcomes of the group’s work will be released today (January 12) in the journal Communications Biology
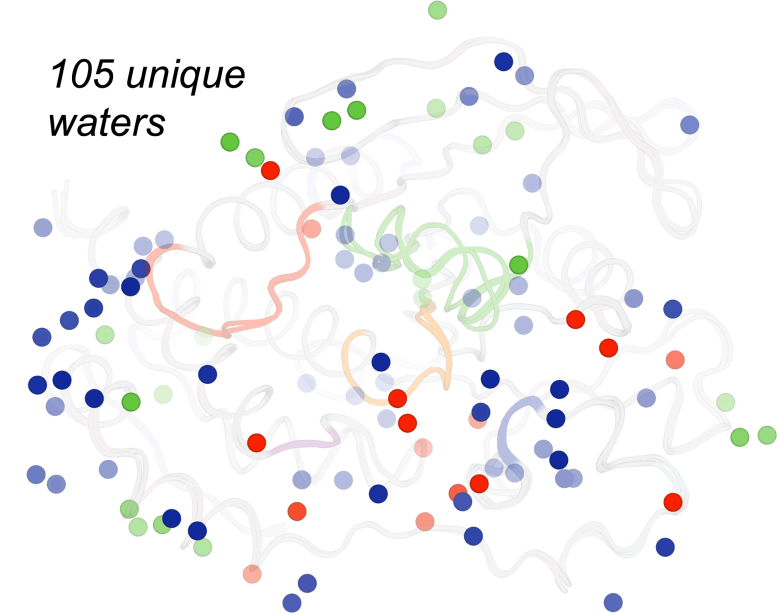
The positions of these water particles are frequently essential for comprehending protein versatility and the capability of drug-like particles to affect protein structure and function. In this research study, various distinct waters appeared at the surface area of the protein under various speculative perturbations such as heat (red), high pressure (green), or default conditions (blue), using complementary insights into these concerns. Credit: Ali Ebrahim & & Liliana Guerrero
Study Insights byDr Daniel Keedy
“Protein structures don’t sit still; they shift between several similar shapes much like a dancer,” stated the research study’s primary private investigator Daniel Keedy,Ph D., a teacher with the CUNY ASRC’s Structural Biology Initiative and a chemistry and biochemistry teacher at The City College of New York and the CUNY GraduateCenter “Unfortunately, existing approaches for viewing proteins only reveal one shape, or suggest the presence of multiple shapes without providing specific details. We wanted to see if different ways of poking at a protein could give a us a more detailed view of how it shape-shifts.”
Experimentation and Observations
For their experiment, the group gotten crystals of action, likewise referred to as PTPN5– a drug target protein for the treatment of a number of illness, consisting of < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>Alzheimer’s</div><div class=glossaryItemBody>Alzheimer's disease is a disease that attacks the brain, causing a decline in mental ability that worsens over time. It is the most common form of dementia and accounts for 60 to 80 percent of dementia cases. There is no current cure for Alzheimer's disease, but there are medications that can help ease the symptoms.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" >Alzheimer’s(*********************** )– and upset them utilizing either high pressure( 2,000 times theEarth’s air pressure) or heat (body temperature level), both of which are extremely various from normal crystallography experiments at air pressure and cryogenic temperature level(-280 ° F,-173 °(*********************** ) C).The scientists saw the samples utilizing X-ray crystallography and observed that heat and high pressure had various impacts on the protein, exposing unique shapes.
Implications for Drug Development
While high pressure isn’t a condition that proteins experience inside the body, Keedy stated the agitation technique exposed various structural states of the protein that might relate to its activity in human cells.
“Having the ability to use perturbations such as heat and pressure to elucidate these different states could give drug developers tools for determining how they can trap a protein in a particular shape using a small-molecule drug to diminish its function,” Keedy included.
Reference: “Pushed to extremes: distinct effects of high temperature versus pressure on the structure of STEP” 12 January 2024, Communications Biology
DOI: 10.1038/ s42003-023-05609 -0





