A current research study exposes that Kallistatin levels increase after weight-loss, using possible brand-new treatments for weight problems and type 2 diabetes through its favorable results on metabolic process and hepatic insulin level of sensitivity.
Following weight-loss, people formerly categorized as obese or overweight display increased levels of the protein Kallistatin in their subcutaneous white fat, according to a current research study carried out by scientists at the DZD.
In addition, Kallistatin enhances metabolic process and might open brand-new restorative choices for individuals with weight problems and type 2 diabetes in the future. The outcomes have actually now been released in Molecular Metabolism
An increasing variety of individuals are establishing type 2 diabetes and weight problems. These are extremely complicated and diverse illness. In order to treat them sustainably, brand-new techniques to treatment are required. Clinical research studies on human beings have actually revealed that greatly obese people produce lessKallistatin Kallistatin is a protein that has numerous results in the body. Among other things, it is associated with neutralizing swelling and recovery injuries.
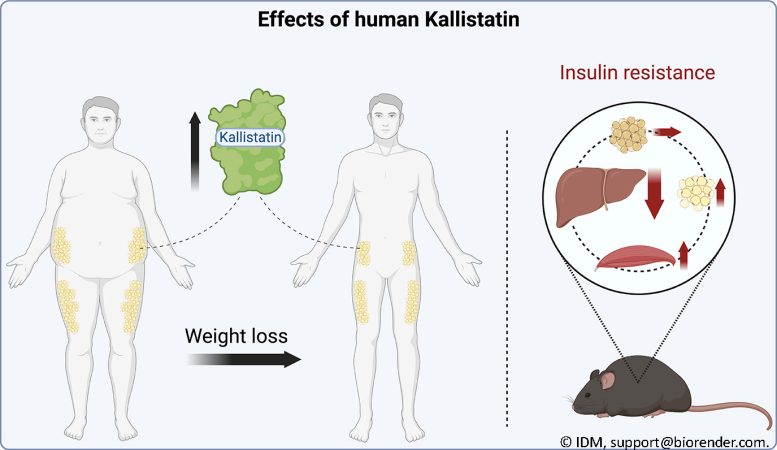
Expression of the protein Kallistatin increases after weight decrease. In mice, it enhances hepatic insulin level of sensitivity. Credit: IDM, [email protected]
The function that Kallistatin plays in glucose metabolic process and its possible viability as a restorative target are presently being examined by scientists from the German Center for Diabetes Research (DZD), the Institute for Diabetes Research and Metabolic Diseases (IDM) of Helmholtz Munich at the Eberhard-Karls-University of Tübingen, and the Department of Diabetology, Endocrinology and Nephrology at the University Hospital Tübingen.
Kallistatin Expression Increases After Weight Loss
To this end, they determined Kallistatin expression in subcutaneous white fat in 47 people with obese to weight problems before and after weight-loss. The outcome: Kallistatin expression increases after weight-loss.
Kallistatin Improves Hepatic Insulin Sensitivity
Additionally, the scientists analyzed the result of the protein in an animal design. In the procedure, they observed that human Kallistatin enhances hepatic < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>insulin</div><div class=glossaryItemBody>Insulin is a hormone that regulates the level of glucose (sugar) in the blood. It is produced by the pancreas and released into the bloodstream when the level of glucose in the blood rises, such as after a meal. Insulin helps to transport glucose from the bloodstream into the cells, where it can be used for energy or stored for later use. Insulin also helps to regulate the metabolism of fat and protein. In individuals with diabetes, their body doesn't produce enough insulin or doesn't respond properly to insulin, leading to high blood sugar levels, which can lead to serious health problems if left untreated.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" > insulin level of sensitivity in diet-induced overweight mice.
“Our results suggest that Kallistatin may be an interesting, yet challenging, therapeutic target for people with obesity and insulin resistance,” states lead authorLeontineSandforth“Because Kallistatin has insulin-sensitizing effects in the liver, it should be investigated as a potential liver-specific target for emulating the beneficial effects of weight loss and potentially treating type 2 diabetes and obesity,” includes last authorProfAndreasBirkenfeld
Reference:“Role of human Kallistatin in glucose and energy homeostasis in mice” by LeontineSandforth,SebastianBrachs, JuliaReinke, DianaWillmes, GencerSancar,Judith Seigner,DavidJuarez-Lopez, ArvidSandforth,Jeffrey D. McBride,Jian-XingMa,Sven Haufe,JensJordan andAndreas L.Birkenfeld,29February2024,MolecularMetabolism
DOI:101016/ j.molmet.2024101905





