Chemists utilize this speculative setup for photochemical responses. Credit: Peter Bellotti
New measurements in natural chemistry through light-mediated synthesis.
A significant objective of natural and medical chemistry in current years has actually been the fast synthesis of three-dimensional particles for the advancement of brand-new drugs. These drug prospects display a range of enhanced residential or commercial properties compared to mainly flat molecular structures, which are shown in scientific trials by greater effectiveness and success rates. However, they might just be produced at terrific cost or not at all utilizing previous techniques.
Chemists led by Prof. Frank Glorius (University of Münster) and his coworkers Prof. M. Kevin Brown (Indiana University Bloomington, U.S.A.) and Prof. Kendall N. Houk (University of California, Los Angeles, U.S.A.) have actually now been successful in transforming numerous classes of flat nitrogen-containing particles into the preferred three-dimensional structures. Using more than 100 unique examples, they had the ability to show the broad applicability of the procedure. This research study has actually now been released in the journal Science.
Light-moderated energy transfer conquers energy barrier
One of the most effective techniques for manufacturing three-dimensional architectures includes the addition of a particle to another, referred to as cycloaddition. In this procedure, 2 brand-new bonds and a brand-new ring are formed in between the particles. For fragrant systems – i.e. flat and especially steady ring substances – this response was not practical with previous techniques. The energy barrier that hinders such a cycloaddition might not be gotten rid of even with the application of heat. For this factor, the authors of the “Science” post checked out the possibility of conquering this barrier through light-mediated energy transfer.
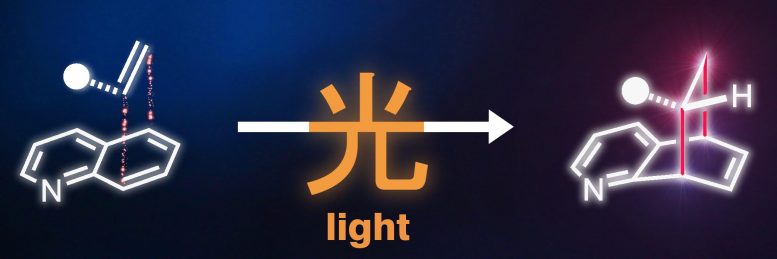
A flat particle including nitrogen is developed into a three-dimensional particle through photochemical synthesis (illustration). The Chinese character on the arrow implies “light.” Credit: Peter Bellotti
“The motif of using light energy to build more complex, chemical structures is also found in nature,” discusses Frank Glorius. “Just as plants use light in photosynthesis to synthesize sugar molecules from the simple building blocks carbon dioxide and water, we use light-mediated energy transfer to produce complex, three-dimensional target molecules from flat basic structures.”
New drug prospects for pharmaceutical applications?
The researchers indicate the “enormous possibilities” of the technique. The unique, non-traditional structural concepts provided by the group in the Science paper will considerably broaden the series of particles that medical chemists can think about in their look for brand-new drugs: for instance, standard foundation including nitrogen and extremely pertinent to pharmaceuticals, such as quinolines, isoquinolines, and quinazolines, which have actually been hardly utilized owing to selectivity and reactivity issues. Through light-mediated energy transfer, they can now be paired with a wide variety of structurally varied alkenes to acquire unique three-dimensional drug prospects or their foundations.
The chemists likewise showed a range of ingenious improvements for the additional processing of these manufactured foundations, utilizing their competence to lead the way for pharmaceutical applications. The technique’s terrific usefulness and the schedule of the needed beginning products are important for the future usage of the innovation: the particles utilized are commercially readily available at low expense or simple to produce.
“We hope that this discovery will provide new impetus in the development of novel medical agents and will also be applied and further investigated in an interdisciplinary manner,” discusses Jiajia Ma. Kevin Brown includes: “Our scientific breakthrough can also gain great significance in the discovery of crop protection agents and beyond.”
Synergy of speculative and computational chemistry
Another unique function of the research study: the researchers clarified the response system and the specific structure of the particles produced for the very first time not just analytically and experimentally in information, however likewise by means of “computational chemistry”: Kendall Houk and Shuming Chen carried out comprehensive computer-aided modeling of the response. They had the ability to demonstrate how these responses work and why they take place really selectively.
“This study is a prime example of the synergy of experimental and computational theoretical chemistry,” highlights Shuming Chen, now a teacher at Oberlin College in Ohio. “Our detailed mechanistic elucidation and understanding of reactivity concepts will enable scientists to develop complementary methods and to use what we learned to design more efficient synthetic routes in the future,” includes Kendall Houk.
The story behind the publication
Using the technique of light-mediated energy transfer, both Jiajia Ma/Frank Glorius (University of Münster) and Renyu Guo/Kevin Brown (Indiana University) had success, separately. Through cooperations with Kendall Houk and Shuming Chen at UCLA, both research study groups found out of the shared discovery. The 3 groups chose to establish their findings even more together in order to share their advancement with the clinical neighborhood as quickly as possible and to offer medical chemists with this innovation to establish unique drugs.
Reference: “Photochemical intermolecular dearomative cycloaddition of bicyclic azaarenes with alkenes” by Jiajia Ma, Shuming Chen, Peter Bellotti, Renyu Guo, Felix Schäfer, Arne Heusler, Xiaolong Zhang, Constantin Daniliuc, M. Kevin Brown, Kendall N. Houk and Frank Glorius, 26 March 2021, Science.
DOI: 10.1126/science.abg0720
Funding: The research study got financial backing from the German Research Foundation (Leibniz Award, Priority Program 2102 and Collaborative Research Centre 858), the European Research Council (H2020 ERC) and the Alfred Krupp von Bohlen-und-Halbach Foundation. On the United States side, the research study was supported by moneying from the National Institutes of Health and the National Science Foundation.





