Filled with briny lakes, the Quisquiro salt flat in South America’s Altiplano represents the sort of landscape that researchers believe might have existed in Gale Crater on Mars. Credit: Maksym Bocharov
By studying the chemical aspects on Mars today — consisting of carbon and oxygen — researchers can work in reverse to piece together the history of a world that as soon as had the conditions required to support life.
Weaving this story, aspect by aspect, from approximately 140 million miles (225 million kilometers) away is a painstaking procedure. But researchers aren’t the type to be quickly prevented. Orbiters and rovers at Mars have actually verified that the world as soon as had liquid water, thanks to hints that consist of dry riverbeds, ancient coastlines, and salted surface area chemistry. Using NASA’s Curiosity Rover, researchers have actually discovered proof for long-lived lakes. They’ve likewise collected natural substances, or life’s chemical foundation. The mix of liquid water and natural substances forces researchers to keep browsing Mars for indications of past — or present — life.
Despite the alluring proof discovered up until now, researchers’ understanding of Martian history is still unfolding, with a number of significant concerns open for argument. For one, was the ancient Martian environment thick adequate to keep the world warm, and hence damp, for the quantity of time required to grow and support life? And the natural substances: are they indications of life — or of chemistry that occurs when Martian rocks connect with water and sunshine?
In a current Nature Astronomy report[1] on a multi-year experiment carried out in the chemistry laboratory inside Curiosity’s stomach, called Sample Analysis at Mars (SAM), a group of researchers uses some insights to assist address these concerns. The group discovered that particular minerals in rocks at Gale Crater might have formed in an ice-covered lake. These minerals might have formed throughout a cold phase sandwiched in between warmer durations, or after Mars lost the majority of its environment and started to turn completely cold.
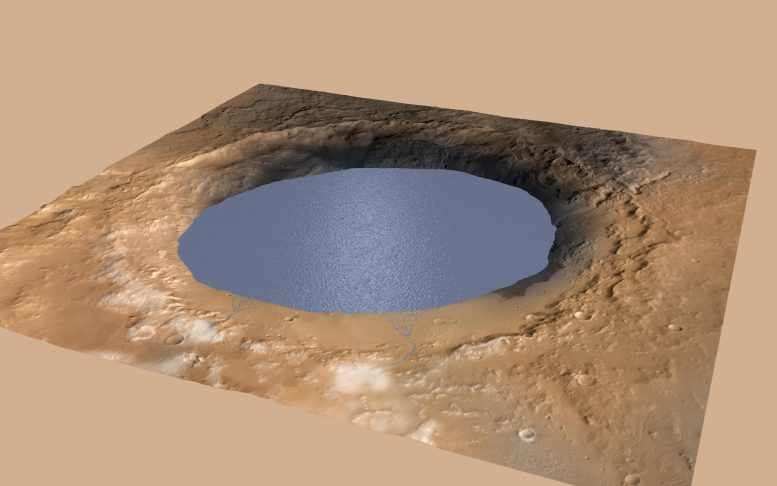
This illustration portrays a lake of water partly filling Mars’ Gale Crater. It would have been filled by overflow from snow melting on the crater’s northern rim. Evidence of ancient streams, deltas, and lakes that NASA’s Curiosity rover has actually discovered in the patterns of sedimentary deposits in Gale recommends the crater held a lake like this another than 3 billion years earlier, filling and drying in several cycles over 10s of countless years. Credit: NASA/JPL-Caltech/ESA/DLR/FU Berlin/MSSS
Gale is a crater the size of Connecticut and Rhode Island integrated. It was chosen as Curiosity’s 2012 landing website since it had indications of previous water, consisting of clay minerals that may assist trap and maintain ancient natural particles. Indeed, while checking out the base of a mountain in the center of the crater, called Mount Sharp, Curiosity discovered a layer of sediments[2] 1,000 feet (304 meters) thick that was transferred as mud in ancient lakes. To form that much sediment an amazing quantity of water would have streamed down into those lakes for millions to 10s of countless warm and damp years, some researchers state. But some geological functions in the crater likewise mean a past that consisted of cold, icy conditions.
“At some point, Mars’ surface environment must have experienced a transition from being warm and humid to being cold and dry, as it is now, but exactly when and how that occurred is still a mystery,” states Heather Franz, a NASA geochemist based at NASA’s Goddard Space Flight Center in Greenbelt, Maryland.
Franz, who led the SAM research study, keeps in mind that aspects such as modifications in Mars’ obliquity and the quantity of volcanic activity might have triggered the Martian environment to alternate in between warm and cold gradually. This concept is supported by chemical and mineralogical modifications[3] in Martian rocks revealing that some layers formed in cooler environments and others formed in warmer ones.
In any case, states Franz, the variety of information gathered by Curiosity up until now recommends that the group is seeing proof for Martian environment modification taped in rocks.
Carbon and oxygen star in the Martian environment story
Franz’s group discovered proof for a cold ancient environment after the SAM laboratory drawn out the gases co2, or CO2, and oxygen from 13 dust and rock samples. Curiosity gathered these samples throughout 5 Earth years.
CO2 is a particle of one carbon atom bonded with 2 oxygen atoms, with carbon functioning as an essential witness when it comes to the mystical Martian environment. In truth, this easy yet flexible aspect is as vital as water in the look for life in other places. On Earth, carbon streams constantly through the air, water, and surface area in a well-understood cycle that depends upon life. For example, plants soak up carbon from the environment in the kind of CO2. In return, they produce oxygen, which people and most other life types utilize for respiration in a procedure that ends with the release of carbon back into the air, once again through CO2, or into the Earth’s crust as life types pass away and are buried.
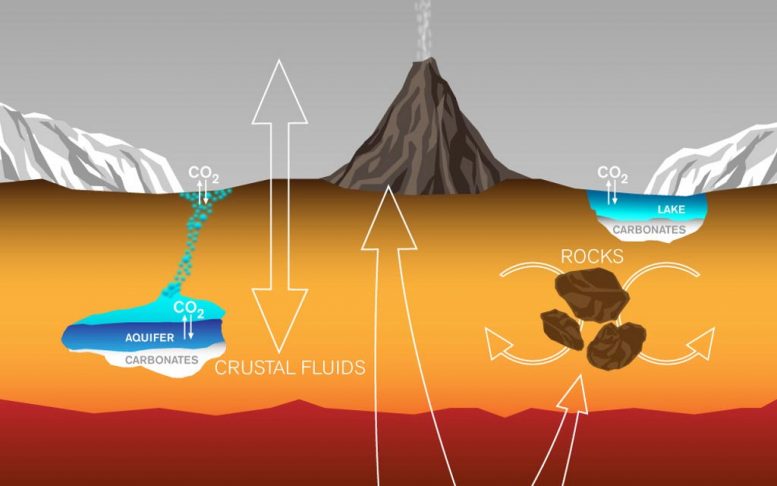
(Click image for complete view.) This graphic portrays courses by which carbon has actually been exchanged amongst Martian interior, surface area rocks, polar caps, waters and environment, and it likewise portrays a system by which it is lost from the environment. Credit: Lance Hayashida/Caltech
Scientists are discovering there’s likewise a carbon cycle on Mars and they’re working to comprehend it. With little water or plentiful surface area life on the Red Planet for a minimum of the previous 3 billion years, the carbon cycle is much various than Earth’s.
“Nevertheless, the carbon cycling is still happening and is still important because it’s not only helping reveal information about Mars’ ancient climate,” states Paul Mahaffy, primary detective on SAM and director of the Solar System Exploration Division at NASA Goddard. “It’s also showing us that Mars is a dynamic planet that’s circulating elements that are the buildings blocks of life as we know it.”
The gases construct a case for a cold duration
After Curiosity fed rock and dust samples into SAM, the laboratory heated every one to almost 1,650 degrees Fahrenheit (900 degrees Celsius) to free the gases inside. By taking a look at the oven temperature levels that launched the CO2 and oxygen, researchers might inform what sort of minerals the gases were originating from. This kind of details assists them comprehend how carbon is biking on Mars.
Various research studies have actually recommended that Mars’ ancient environment, consisting of mainly CO2, might have been thicker than Earth’s is today. Most of it has actually been lost to area, however some might be saved in rocks at the world’s surface area, especially in the kind of carbonates, which are minerals made from carbon and oxygen. On Earth, carbonates are produced when CO2 from the air is soaked up in the oceans and other bodies of water and after that mineralized into rocks. Scientists believe the very same procedure occurred on Mars which it might assist discuss what occurred to a few of the Martian environment.
Yet, objectives to Mars haven’t discovered enough carbonates in the surface area to support a thick environment.
Nonetheless, the couple of carbonates that SAM did spot exposed something intriguing about the Martian environment through the isotopes of carbon and oxygen saved in them. Isotopes are variations of each aspect that have various masses. Because various chemical procedures, from rock development to biological activity, utilize these isotopes in various percentages, the ratios of heavy to light isotopes in a rock offer researchers with hints to how the rock formed.
In a few of the carbonates SAM discovered, researchers discovered that the oxygen isotopes were lighter than those in the Martian environment. This recommends that the carbonates did not form long earlier merely from climatic CO2 soaked up into a lake. If they had, the oxygen isotopes in the rocks would have been a little much heavier than the ones in the air.
While it’s possible that the carbonates formed extremely early in Mars’ history, when the climatic structure was a bit various than it is today, Franz and her coworkers recommend that the carbonates most likely formed in a freezing lake. In this situation, the ice might have drawn up heavy oxygen isotopes and left the lightest ones to form carbonates later on. Other Curiosity researchers[4] have actually likewise provided proof recommending that ice-covered lakes might have existed in Gale Crater.
So where is all the carbon?
The low abundance of carbonates on Mars is perplexing, researchers state. If there aren’t a number of these minerals at Gale Crater, maybe the early environment was thinner than anticipated. Or perhaps something else is keeping the missing out on climatic carbon.
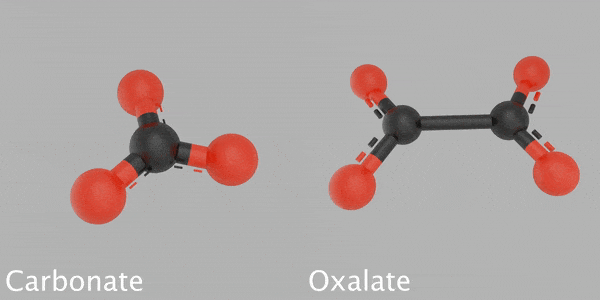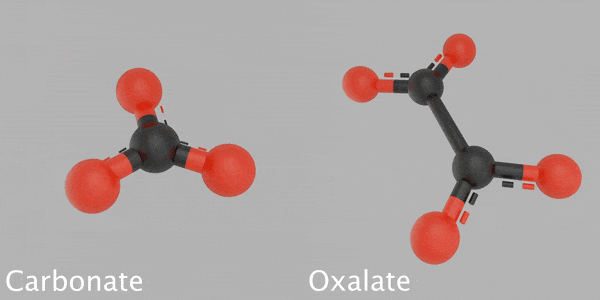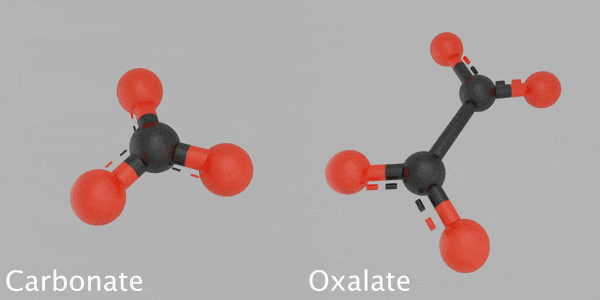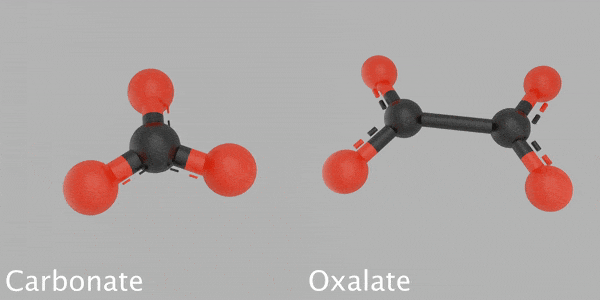
This animated image reveals a 3D design of a carbonate particle beside a 3D design of an oxalate particle. The carbonate is made from a carbon atom that’s bonded with 3 oxygen atoms. The oxalate is made from 2 carbon atoms bonded with 4 oxygen atoms. Credit: James Tralie/NASA/Goddard Space Flight Center
Based on their analysis, Franz and her coworkers recommend that some carbon might be sequestered in other minerals, such as oxalates, which keep carbon and oxygen in a various structure than carbonates. Their hypothesis is based upon the temperature levels at which CO2 was launched from some samples inside SAM — too low for carbonates, however perfect for oxalates — and on the various carbon and oxygen isotope ratios than the researchers saw in the carbonates.
Oxalates are the most typical kind of natural mineral produced by plants on Earth. But oxalates likewise can be produced without biology. One method is through the interaction of climatic CO2 with surface area minerals, water, and sunshine, in a procedure referred to as abiotic photosynthesis. This kind of chemistry is difficult to discover on Earth since there’s plentiful life here, however Franz’s group wants to produce abiotic photosynthesis in the laboratory to determine if it in fact might be accountable for the carbon chemistry they’re seeing in Gale Crater.
On Earth, abiotic photosynthesis might have led the way for photosynthesis amongst a few of the very first tiny life types, which is why discovering it on other worlds interests astrobiologists.
Even if it ends up that abiotic photosynthesis locked some carbon from the environment into rocks at Gale Crater, Franz and her coworkers wish to study soil and dust from various parts of Mars to comprehend if their arise from Gale Crater show a worldwide photo. They might one day get a possibility to do so. NASA’s Perseverance Mars rover, due to release to Mars in between July and August 2020, prepares to evacuate samples in Jezero Crater for possible go back to laboratories on Earth.
References:
- “Indigenous and exogenous organics and surface–atmosphere cycling inferred from carbon and oxygen isotopes at Gale crater” by H. B. Franz, P. R. Mahaffy, C. R. Webster, G. J. Flesch, E. Raaen, C. Freissinet, S. K. Atreya, C. H. House, A. C. McAdam, C. A. Knudson, P. D. Archer Jr., J. C. Stern, A. Steele, B. Sutter, J. L. Eigenbrode, D. P. Glavin, J. M. T. Lewis, C. A. Malespin, M. Millan, D. W. Ming, R. Navarro-González and R. E. Summons, 27 January 2020, Nature Astronomy.
DOI: 10.1038/s41550-019-0990-x - “Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars” by J. P. Grotzinger, S. Gupta, M. C. Malin, D. M. Rubin, J. Schieber, K. Siebach, D. Y. Sumner, K. M. Stack, A. R. Vasavada, R. E. Arvidson, F. Calef III, L. Edgar, W. F. Fischer, J. A. Grant, J. Griffes, L. C. Kah, M. P. Lamb, K. W. Lewis, N. Mangold, M. E. Minitti, M. Palucis, M. Rice, R. M. E. Williams, R. A. Yingst, D. Blake, D. Blaney, P. Conrad, J. Crisp, W. E. Dietrich, G. Dromart, K. S. Edgett, R. C. Ewing, R. Gellert, J. A. Hurowitz, G. Kocurek, P. Mahaffy, M. J. McBride, S. M. McLennan, M. Mischna, D. Ming, R. Milliken, H. Newsom, D. Oehler, T. J. Parker, D. Vaniman, R. C. Wiens and S. A. Wilson, 9 October 2015, Science.
DOI: 10.1126/science.aac7575 - “Chemical alteration of fine-grained sedimentary rocks at Gale crater” by N. Mangolda, E. Dehouck, C. Fedo, O. Forni, C. Achilles, T. Bristow, R. T. Downs, J. Frydenvang, O. Gasnault, J. L’Haridon, L. Le Deit, S. Maurice, S. M. McLennan, P.-Y. Meslin, S. Morrison, H. E. Newsom, E. Rampe, W. Rapin, F. Rivera-Hernandez, M. Salvatore and R. C. Wiens, 17 November 2018, Icarus.
DOI: 10.1016/j.icarus.2018.11.004 - “Subsistence of ice-covered lakes during the Hesperian at Gale crater, Mars” by Alexandre M. Kling, Robert M. Haberle, Christopher P. McKay, Thomas F. Bristow and Frances Rivera-Hernández, 9 November 2019, Icarus.
DOI: 10.1016/j.icarus.2019.113495





