Researchers have actually found that modifications in gut microbiome, particularly a decrease in Clostridia germs due to prescription antibiotics and a high-fat diet plan, can result in sorbitol intolerance. This intolerance manifests as indigestion from taking in sorbitol, a typical sugar alcohol in numerous foods and items. They discovered that managing gut oxygen levels with particular germs or drugs like mesalazine might bring back the capability to absorb sorbitol, recommending a brand-new treatment technique for sorbitol intolerance.
Gut microorganisms missing out on due to prescription antibiotics and a high-fat diet plan might be accountable for ‘sorbitol intolerance’.
Scientists at UC Davis have actually found changes in the gut microbiome that result in problems in absorbing sorbitol.
Sorbitol, a sugar alcohol, is utilized in sugar-free gum, mints, sweet and other items. It is likewise discovered naturally in apricots, apples, pears, avocadoes, and other foods. At high levels, sorbitol can trigger bloating, cramps, and diarrhea. For some individuals, even a percentage triggers indigestion, a condition referred to as sorbitol intolerance.
A brand-new research study with mice discovered that taking prescription antibiotics, integrated with a high-fat diet plan, decreased the variety of Clostridia gut microorganisms, which can break down sorbitol. The findings were released in the journal Cell
“Our research suggests that microbial sorbitol degradation normally protects the host against sorbitol intolerance. However, an impairment in the microbial ability to break down sorbitol causes sorbitol intolerance,” stated Jee-Yon Lee, very first author of the research study. Lee is an assistant job researcher in the UC Davis Department of Medical Microbiology and Immunology.
How oxygen levels in the gut impact microorganisms
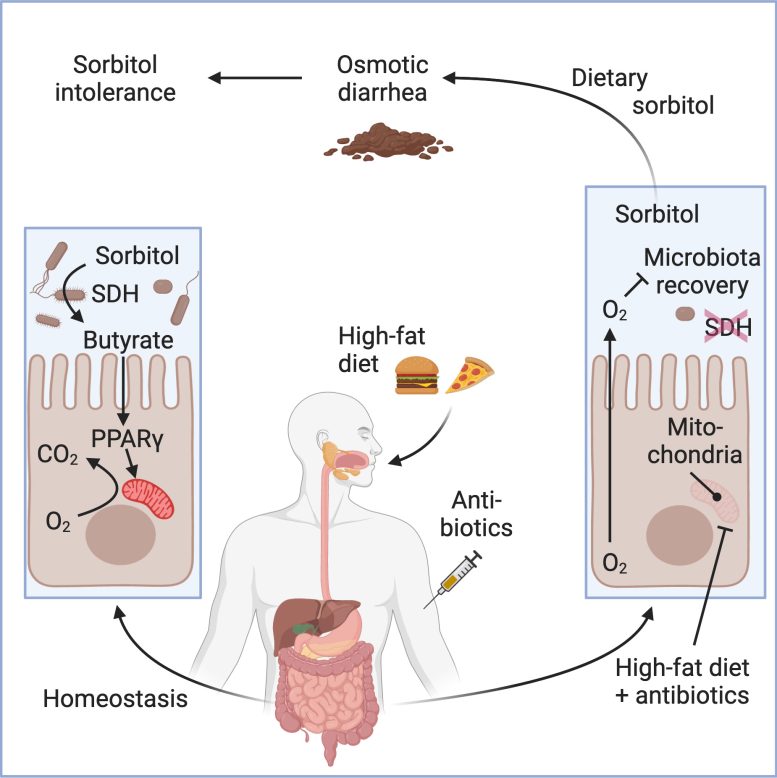
The scientists utilized metagenomic analysis to determine which gut germs have genes that make the enzyme that breaks down sorbitol. They likewise determined which of those gut germs abounded previously– however not after– antibiotic treatment.
This analysis enabled them to absolutely no in on gut microorganisms coming from the classClostridium Clostridium are anaerobic, suggesting they do not like environments with oxygen.
The scientists discovered that after the mice were offered prescription antibiotics and fed a diet plan high in hydrogenated fat, the cells lining the gut utilized less oxygen. This produced a greater level of oxygen in the gut, reducingClostridia Without enough Clostridia, sorbitol was not broken down in the gut.
The scientists carried out a number of experiments to attempt to bring back the gut germs so it might break down sorbitol once again.
Taking prescription antibiotics, integrated with a high-fat diet plan, decreased the variety of Clostridia gut microorganisms. Image produced with BioRender. Credit: UC Davis Health
In one, they fed the mice Anaerostipes caccae, a gut germs that produces butyrate. Butyrate is a short-chain fatty < period class =(****************************************************** )aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>acid</div><div class=glossaryItemBody>Any substance that when dissolved in water, gives a pH less than 7.0, or donates a hydrogen ion.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" > acid produced as part of the regular fermentation procedure in the gut.(***************************************************************************************************************************************** )improves oxygen use by the cells that line the gut, the epithelial lining, which minimizes oxygen levels in the big intestinal tract.
Regulating the oxygen level withAnaerostipes caccae brought back the regular levels ofClostridia, which safeguarded the mice from sorbitol-induced diarrhea, even after the butyrate-producing germs had actually been cleared from the mouse’s gastrointestinal system.
The scientists recommend that a substance abuse to deal with ulcerative colitis,Crohn’s illness, and other inflammatory bowel illness, mesalazine( 5-aminosalicylate), might be a treatment for sorbitol intolerance in people. Mesalazine, likewise referred to as mesalamine, works likewise to the butyrate-producing germs, bring back the low oxygen levels in the intestinal tract chosen by Clostridia.
“This discovery is crucial, given the prevalent use of sorbitol and similar sugar alcohols in the production of keto-friendly diet foods that are high in fat content,” Lee stated. “It also highlights the importance of oxygen consumption by the epithelial lining in the intestines in maintaining a healthy balance of gut bacteria, especially Clostridia, for proper digestion of certain sugars.”
An essential restriction of the research study is that mice can endure much greater sorbitol levels than people. Mice have a cecum– a pouch in their gastrointestinal system that slows the circulation of digestive contents and assists absorb carbs, which might add to having the ability to much better endure sorbitol. Clinical research studies will be required to check the hypothesis that mesalazine might deal with sorbitol intolerance in people.
“Our study provides a completely new starting point for approaches to diagnose, prevent, and treat sorbitol intolerance,” stated Andreas Bäumler, senior author of the research study. Bäumler is a prominent teacher and vice chair of research study in the UC Davis Department of Medical Microbiology and Immunology.
Reference: “High fat intake sustains sorbitol intolerance after antibiotic-mediated Clostridia depletion from the gut microbiota” by Jee-Yon Lee, Connor R. Tiffany, Scott P. Mahan, Matthew Kellom, Andrew W.L. Rogers, Henry Nguyen, Eric T. Stevens, Hugo L.P. Masson, Kohei Yamazaki, Maria L. Marco, Emiley A. Eloe-Fadrosh, Peter J. Turnbaugh and Andreas J. Bäumler, 15 February 2024, Cell
DOI: 10.1016/ j.cell.202401029
Co- authors consist of Connor Tiffany, Scott Mahan, Andrew Rogers, Henry Nguyen, and Hugo Masson of the UC Davis School of Medicine; Eric Stevens and Maria Marco of UC Davis; Matthew Kellom and Emiley A. Eloe-Fadrosh of Lawrence Berkeley National Laboratory; Kohei Yamazak of Kitasato University in Japan; and Peter Turnbaugh of UC San Francisco (UCSF) and Chan Zuckerberg Biohub.
The research study was moneyed by the Kenneth Rainin Foundation.





