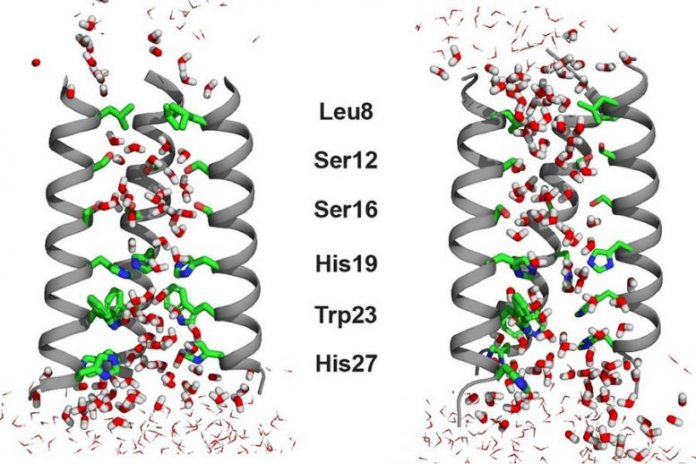
Different water characteristics are seen in between the closed (left) and open (ideal) states of the transmembrane proton channel of the influenza B infection M2 protein. Water particles are a little more oriented outdoors state than in the closed state to enable “proton hopping” by means of the water’s hydrogen bonds. Credit: Image thanks to the scientists
Research on how water acts in a proton channel supplies possible brand-new opportunities for influenza treatment.
In a brand-new research study of water characteristics, a group of MIT chemists led by Professor Mei Hong, in partnership with Associate Professor Adam Willard, has actually found that water in an ion channel is anisotropic, or partly lined up. The scientists’ information, the very first of their kind, show the relation of water characteristics and order to the conduction of protons in an ion channel. The work likewise supplies possible brand-new opportunities for the advancement of antiviral drugs or other treatments.
Members of the Hong laboratory carried out advanced nuclear magnetic resonance (NMR) experiments to show the presence of anisotropic water in the proton channel of the influenza M infection, while members of the Willard group performed independent all-atom molecular characteristics simulations to confirm and enhance the speculative information. Their research study, of which Hong was the senior author, was released in Communications Biology, and was co-authored by Martin Gelenter, Venkata Mandala, and Aurelio Dregni of the Hong Lab, and Michiel Niesen and Dina Sharon of the Willard group.
Channel water and influenza infection
The influenza B infection protein BM2 is a protein channel that acidifies the infection, assisting it to launch its hereditary product into contaminated cells. The water in this channel plays an important function in assisting the influenza infection end up being transmittable, since it helps with proton conduction inside the channel to cross the lipid membrane.
Previously, Hong’s laboratory studied how the amino acid histidine shuttles protons from water into the influenza infection, however they hadn’t examined the water particles themselves in information. This brand-new research study has actually offered the missing out on link in a complete understanding of the combined hydrogen-bonded chain in between water and histidine inside the M2 channel. To suppress the influenza infection protein, the channel would need to be plugged with little particles — i.e., antiviral drugs — so that the water path would be broken.
In order to line up the water-water hydrogen bonds for “proton hopping,” water particles need to be at least partly oriented. However, to experimentally find the small quantity of recurring positioning of water particles in a channel, without freezing the sample, is exceptionally tough. As an outcome, most of previous research studies on the subject were carried out by computational chemists like Willard. Experimental information on this subject were generally limited to crystal structures gotten at cryogenic temperature levels. The Hong laboratory embraced a relaxation NMR strategy that can be utilized at the much balmier temperature level of around 0 degrees Celsius. At this temperature level, the water particles turned simply gradually adequate for the scientists to observe the movement and recurring orientation in the channel for the very first time.
More area, more order
The proof yielded by Hong’s NMR experiments suggested that the water particles outdoors state of the BM2 channel are more lined up than they remain in the closed state, although there are a lot more water particles outdoors state. The scientists found this recurring order by determining a magnetic home called chemical shift anisotropy for the water protons. The greater water positioning at low pH came as a surprise.
“This was initially counterintuitive to us,” states Hong. “We know from a lot of previous NMR data that the open channel has more water molecules, so one would think that these water molecules should be more disordered and random in the wider channel. But no, the waters are actually slightly better aligned based on the relaxation NMR data.” Molecular vibrant simulations suggested that this order is caused by the crucial proton-selective residue, a histidine, which is favorably charged at low pH.
By using solid-state NMR spectroscopy and molecular characteristics simulations, the scientists likewise discovered that water turned and equated throughout the channel more quickly in the low-pH open state than in the high-pH closed state. These results together suggest that the water particles go through small-amplitude reorientations to develop the positioning that is needed for proton hopping.
Inhibiting proton conduction, obstructing the infection
By utilizing molecular characteristics simulations carried out by Willard and his group, the scientists had the ability to observe that the water network has less hydrogen-bonding traffic jams outdoors state than in the closed state. Thus, quicker characteristics and greater orientational order of water particles outdoors channel develop the water network structure that is needed for proton hopping and effective infection on the infection’ part.
When an influenza infection gets in a cell, it enters into a little compartment called the endosome. The endosome compartment is acidic, which activates the protein to open its water-permeated path and carry out the protons into the infection. Acidic pH has a high concentration of hydrogen ions, which is what the M2 protein performs. Without the water particles passing on the protons, the protons will not reach the histidine, an important amino acid residue. The histidine is the proton-selective residue, and it turns in order to shuttle bus the protons brought by the water particles. The relay chain in between the water particles and the histidine is for that reason accountable for proton conduction through the M2 channel. Therefore, the findings suggested in this research study might show pertinent to the advancement of antiviral drugs and other useful applications.
Reference: “Water orientation and dynamics in the closed and open influenza B virus M2 proton channels” by Martin D. Gelenter, Venkata S. Mandala, Michiel J. M. Niesen, Dina A. Sharon, Aurelio J. Dregni, Adam P. Willard and Mei Hong, 12 March 2021, Communications Biology.
DOI: 10.1038/s42003-021-01847-2