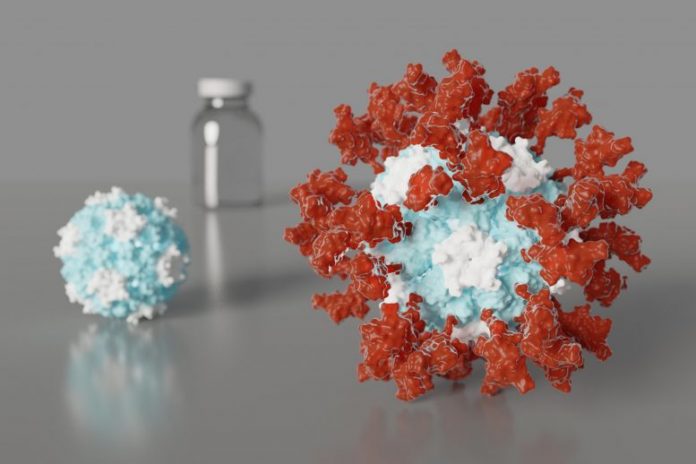
Artist’s representation of an ultrapotent COVID-19 vaccine prospect in which 60 pieces of a coronavirus protein (red) embellish nanoparticles (blue and white). The vaccine prospect was created utilizing techniques established at the UW Medicine Institute for Protein Design. The molecular structure of the vaccine approximately imitates that of an infection, which might represent its improved capability to provoke an immune reaction. Credit: Ian Haydon/ UW Medicine Institute for Protein Design
Preclinical information released in Cell reveal the nanoparticle vaccine stimulates exceptionally high levels of protective antibodies in animal designs.
An ingenious nanoparticle vaccine prospect for the pandemic coronavirus produces virus-neutralizing antibodies in mice at levels 10 times higher than is seen in individuals who have actually recuperated from COVID-19 infections. Designed by researchers at the University of Washington School of Medicine in Seattle, the vaccine prospect has actually been moved to 2 business for scientific advancement.
Compared to vaccination with the soluble SARS-CoV-2 Spike protein, on which lots of leading COVID-19 vaccine prospects are based, the brand-new nanoparticle vaccine produced 10 times more reducing the effects of antibodies in mice, even at a sixfold lower dosage. The information likewise reveal a strong B-cell reaction after immunization, which can be crucial for immune memory and a long lasting vaccine impact. When administered to a single nonhuman primate, the nanoparticle vaccine produced reducing the effects of antibodies targeting numerous various websites on the Spike protein. Researchers state this might make sure security versus altered pressures of the infection, ought to they develop. The Spike protein becomes part of the coronavirus infectivity equipment.
The findings were released on October 30, 2020, in Cell. The lead authors of this paper are Alexandra Walls, a research study researcher in the lab of David Veesler, a UW partner teacher of biochemistry; and Brooke Fiala, a research study researcher in the laboratory of Neil King, UW assistant teacher of biochemistry.
The vaccine prospect was established utilizing structure-based vaccine style strategies created at UW Medicine. It is a self-assembling protein nanoparticle that shows 60 copies of the SARS-CoV-2 Spike protein’s receptor-binding domain in an extremely immunogenic selection. The molecular structure of the vaccine approximately imitates that of an infection, which might represent its improved capability to provoke an immune reaction.
“We hope that our nanoparticle platform may help fight this pandemic that is causing so much damage to our world,” stated King, developer of the computational vaccine style innovation at the Institute for Protein Design. “The potency, stability, and manufacturability of this vaccine candidate differentiate it from many others under investigation.”
Hundreds of prospect vaccines for COVID-19 remain in advancement around the globe. Many need big dosages, intricate production, and cold-chain shipping and storage. An ultrapotent vaccine that is safe, reliable at low dosages, basic to produce and steady beyond a freezer might allow vaccination versus COVID-19 on an international scale.
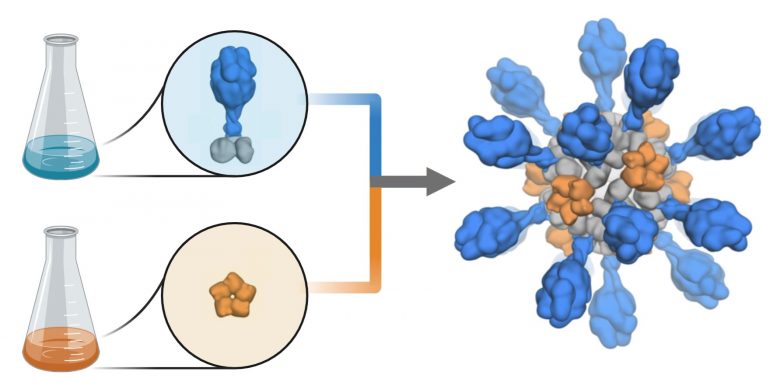
Production schematic demonstrate how coronavirus proteins are contributed to a computer-designed nanoparticle platform to produce a prospect vaccine versus COVID-19. The vaccine prospect was created and evaluated in animal designs by scientists at the University of Washington School of Medicine. Credit: Ian Haydon/UW Medicine Institute for Protein Design
“I am delighted that our studies of antibody responses to coronaviruses led to the design of this promising vaccine candidate,” stated Veesler, who led the idea of a multivalent, receptor-binding, domain-based vaccine.
The lead vaccine prospect from this report is being certified non-exclusively and royalty-free throughout the pandemic by the University of Washington. One licensee, Icosavax, a Seattle biotechnology business co-founded in 2019 by King, is presently advancing research studies to support regulative filings and has actually started the U.S. Food and Drug Administration’s Good Manufacturing Practice. To speed up development by Icosavax to the center, Amgen has actually accepted make a crucial intermediate for these preliminary scientific research studies. Another licensee, SK bioscience of South Korea, is advancing its own research studies to support scientific and additional advancement.
Reference: “Elicitation of powerful reducing the effects of antibody actions by created protein nanoparticle vaccines for SARS-CoV-2Alexandra C. Walls, Brooke Fiala, Alexandra Schäfer, Samuel Wrenn, Minh N. Pham, Michael Murphy, Longping V. Tse, Laila Shehata, Megan A. O’Connor, Chengbo Chen, Mary Jane Navarro, Marcos C. Miranda, Deleah Pettie, Rashmi Ravichandran, John C. Kraft, Cassandra Ogohara, Anne Palser, Sara Chalk, E-Chiang Lee, Kathryn Guerriero, Elizabeth Kepl, Cameron M. Chow, Claire Sydeman, Edgar A. Hodge, Brieann Brown, Jim T. Fuller, Kenneth H. Dinnon, III, Lisa E. Gralinski, Sarah R. Leist, Kendra L. Gully, Thomas B. Lewis, Miklos Guttman, Helen Y. Chu, Kelly K. Lee, Deborah H. Fuller, Ralph S. Baric, Paul Kellam, Lauren Carter, Marion Pepper, Timothy P. Sheahan, David Veesler and Neil P. King, Accepted 26 October 2020, Cell.
DOI: 10.1016/j.cell.2020.10.043
The research study reported in Cell was supported by the National Institute of Allergy and Infectious Diseases (DP1AI158186, HHSN272201700059C, 3U01AI42001-02S1), National Institute of General Medical Sciences (R01GM120553, R01GM099989), Bill & Melinda Gates Foundation (OPP1156262, OPP1126258, OPP1159947), Defense Threat Reduction Agency (HDTRA1-18-1-0001), Pew Biomedical Scholars Award, Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, Fast Grants, Animal Models Contract HHSN272201700036I-75N93020F00001, University of Washington’s Proteomics Resource (UWPR95794), North Carolina Coronavirus Relief Fund, and presents from Jodi Green and Mike Halperin and from The Audacious Project.