Researchers at Johns Hopkins Medicine have actually discovered a brand-new function of the SYNGAP1 gene in memory and knowing, revealing its significance beyond enzyme activity to consist of scaffolding functions at synapses. This finding, which exposes the gene’s double function in managing synaptic strength and plasticity, might result in much better treatments for kids with neurodevelopmental conditions connected to SYNGAP1 anomalies.
Research on mice might direct the pursuit of treatments for brain advancement conditions in kids with anomalies in the SYNGAP1 gene.
Neuroscientists at Johns Hopkins Medicine have actually found a formerly unidentified function of the SYNGAP1 gene, a < period class =(******************************************** )aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>DNA</div><div class=glossaryItemBody>DNA, or deoxyribonucleic acid, is a molecule composed of two long strands of nucleotides that coil around each other to form a double helix. It is the hereditary material in humans and almost all other organisms that carries genetic instructions for development, functioning, growth, and reproduction. Nearly every cell in a person’s body has the same DNA. Most DNA is located in the cell nucleus (where it is called nuclear DNA), but a small amount of DNA can also be found in the mitochondria (where it is called mitochondrial DNA or mtDNA).</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" > DNA series that manages memory and knowing in mammals, consisting of mice and people.
(************** )(******************************************************************************************************* )finding, just recently released inScience, might impact the advancement of treatments developed for kids with SYNGAP1 anomalies, who have a series of neurodevelopmental conditions marked by intellectual special needs, autistic-like habits, and epilepsy.
In basic, SYNGAP1, in addition to other genes, control knowing and memory by making proteins that control the strength of synapses– the connections in between brain cells.
Previously, the scientists state, the SYNGAP1 gene was believed to work solely by encoding a protein that acts like an enzyme, managing chain reactions that result in modifications in the strength of synapses.Now, the researchers state, their experiments in mice reveal that protein encoded by the gene might likewise operate more like a so-called scaffolding protein that controls synaptic plasticity, or how synapses get more powerful or weaker with time, independent of its enzyme activity.The SynGAP protein appears to function as a traffic supervisor, they state, directing where and what brain proteins are at synapses.
Discovery and Experimentation
With his group, Richard Huganir,Ph D., Bloomberg Distinguished Professor of Neuroscience and Psychological and Brain Sciences and director of the Solomon H. Snyder Department of Neuroscience at the Johns Hopkins University School of Medicine, initially separated the SYNGAP1 gene in 1998.
SynGAP proteins are extremely plentiful at the < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>synapse</div><div class=glossaryItemBody>A synapse is a specialized junction between nerve cells that allows for the transfer of electrical or chemical signals, through the release of neurotransmitters by the presynaptic neuron and the binding of receptors on the postsynaptic neuron. It plays a key role in communication between neurons and in various physiological processes including perception, movement, and memory.</div>" data-gt-translate-attributes ="(** )" tabindex ="0" function ="link" > synapse, statesHuganir, and it has actually long been believed that SynGAP’s primary function was to stimulate enzymatic chain reaction that control synapse strength.
But, dealing with the SynGAP protein,Huganir and others had actually started to see that SynGAP proteins have a weird residential or commercial property when they connect with the significant synaptic scaffolding protein, PSD-95They change into liquid beads.
“For an enzymatic protein, that structural transformation is unusual,” states(************************************************************************************************************************************************************ ).
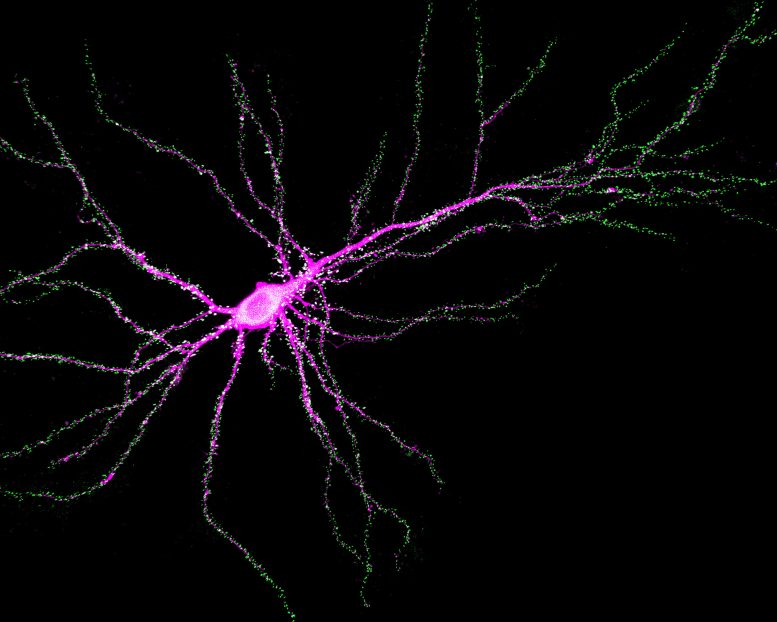
Neuron revealing SynGAP( green) binding to PSD-95 at synapses.Credit:YoichiAraki andRickHuganir,JohnsHopkinsMedicine
To tease out and comprehend the function of SynGAP’s strange liquid change,Huganir, neuroscience trainerYoichiAraki and Huganir’s research study group atJohnsHopkins developed experiments in nerve cells in which they placed anomalies in the so-called space domain of the SYNGAP1 gene that would get rid of the enzymatic function of SynGAP without impacting its structure.
TheJohns(************************************************************************************************************************************************************** )group discovered that, even without the enzymatic activity, the synapse worked usually, recommending that the structural residential or commercial property alone is extremely essential for SynGAP function.
(******************************************************************************************************* )research study group next did the very same kind of genetic modification in mice to get rid of the enzymatic function of SynGAP, and discovered comparable outcomes: Synapses acted usually, without any issues in synaptic plasticity, and the mice had no trouble in knowing and memory habits. The research study group states this shows that SynGAP’s structural residential or commercial property sufficed for typical cognitive habits.
SynGAP’s Dual Function and Implications for Therapy
To comprehend how SynGAP’s structure controls synapses, the researchers evaluated synapses more carefully to discover that SynGAP protein took on the binding of AMPA receptor/TARP complexes, a package of neurotransmitter proteins that reinforce synapses, and the PSD-95 scaffolding protein.
The experiments recommend that, at rest, SynGAP firmly binds to PSD-95, not permitting it to bind to any other proteins in the synapse. However, throughout synaptic plasticity, discovering, and memory, SynGAP protein detached from PSD-95, left the synapse, and enabled neurotransmitter receptor complexes to bind to PSD-95 This made the synapse more powerful and increased transmission in between brain cells.
“This sequence happens without the catalytic activity typical of SynGAP,” statesHuganir Rather, SynGAP corrals PSD-95 when bound to it, however when SynGAP leaves this synapse, PSD-95 is open to bind to AMPA receptor/TARP complexes.
In kids with SynGAP anomalies, about half the variety of SynGAP proteins remain in the synapse. With less SynGAP proteins, PSD-95 might bind more with the AMPA receptor/TARP complexes, altering neuronal connections and developing the increased brain cell activity attribute of epileptic seizures typical amongst kids with SynGAP anomalies.
Huganir states that both functions of SynGAP– enzymatic and the “traffic management” action of a scaffolding protein– might now be essential in discovering treatments for SynGAP-related neurodevelopmental conditions. Their research study likewise recommends that targeting simply one function of SynGAP alone might not suffice to have a substantial effect.
Reference: “SynGAP regulates synaptic plasticity and cognition independently of its catalytic activity” by Yoichi Araki, Kacey E. Rajkovich, Elizabeth E. Gerber, Timothy R. Gamache, Richard C. Johnson, Thanh Hai N. Tran, Bian Liu, Qianwen Zhu, Ingie Hong, Alfredo Kirkwood and Richard Huganir, 1 March 2024, Science
DOI: 10.1126/ science.adk1291
In addition to Araki and Huganir, Johns Hopkins researchers who authored the report on the research study are Kacey Rajkovich, Elizabeth Gerber, Timothy Gamache, Richard Johnson, Thanh Hai Tran, Bian Liu, Qianwen Zhu, Ingie Hong and Alfredo Kirkwood.
Funding for the research study was supplied by the < period class ="glossaryLink" aria-describedby ="tt" data-cmtooltip ="<div class=glossaryItemTitle>National Institutes of Health</div><div class=glossaryItemBody>The National Institutes of Health (NIH) is the primary agency of the United States government responsible for biomedical and public health research. Founded in 1887, it is a part of the U.S. Department of Health and Human Services. The NIH conducts its own scientific research through its Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program. With 27 different institutes and centers under its umbrella, the NIH covers a broad spectrum of health-related research, including specific diseases, population health, clinical research, and fundamental biological processes. Its mission is to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability.</div>" data-gt-translate-attributes="[{"attribute":"data-cmtooltip", "format":"html"}]" tabindex ="0" function ="link" >NationalInstitutes ofHealth( R01 MH112151, R01 NS036715, T32 MH015330) and the SynGAPResearchFund