A brand-new research study is drawing the most in-depth photo yet of SARS-CoV-2 infection in the lung, exposing systems that lead to deadly COVID-19, and might discuss long-lasting problems and demonstrate how COVID-19 varies from other contagious illness.
Led by scientists at Columbia University Vagelos College of Physicians and Surgeons and Herbert Irving Comprehensive Cancer Center, the research study discovered that in clients who passed away of the infection, COVID-19 let loose a harmful trifecta of runaway swelling, direct damage and impaired regrowth of lung cells associated with gas exchange, and sped up lung scarring.
Though the research study took a look at lungs from clients who had actually passed away of the illness, it offers strong leads regarding why survivors of serious COVID might experience long-lasting breathing problems due to lung scarring.
“It’s a devastating disease, but the picture we’re getting of the COVID-19 lung is the first step towards identifying potential targets and therapies that disrupt some of the disease’s vicious circuits. In particular, targeting cells responsible for pulmonary fibrosis early on could possibly prevent or ameliorate long-term complications in survivors of severe COVID-19,” states Benjamin Izar, MD, PhD, assistant teacher of medication, who led a group of more than 40 detectives to finish in numerous months a series of analyses that normally takes years.
This research study and a buddy paper led by scientists at Harvard/MIT, to which the Columbia detectives likewise contributed, were released in the journal Nature on April 29.
Study develops atlas of cells in COVID lung
The brand-new research study is special from other examinations because it straight takes a look at lung tissue (instead of sputum or bronchial washes) utilizing single-cell molecular profiling that can determine each cell in a tissue sample and record each cell’s activity, leading to an atlas of cells in COVID lung.
“A normal lung will have many of the same cells we find in COVID, but in different proportions and different activation states,” Izar states. “In order to understand how COVID-19 is different compared to both control lungs and other forms of infectious pneumonias, we needed to look at thousands of cells, one by one.”
Izar’s group took a look at the lungs of 19 people who passed away of COVID-19 and went through quick autopsy (within hours of death)—throughout which lung and other tissues were gathered and right away frozen—and the lungs of non-COVID-19 clients. In cooperation with detectives at Cornell University, the scientists likewise compared their findings to lungs of clients with other breathing health problems.
Drugs targeting IL-1ß might lower swelling
Compared to typical lungs, lungs from the COVID clients were filled with immune cells called macrophages, the research study discovered.
Typically throughout an infection, these cells chew up pathogens however likewise manage the strength of swelling, which likewise assists in the battle.
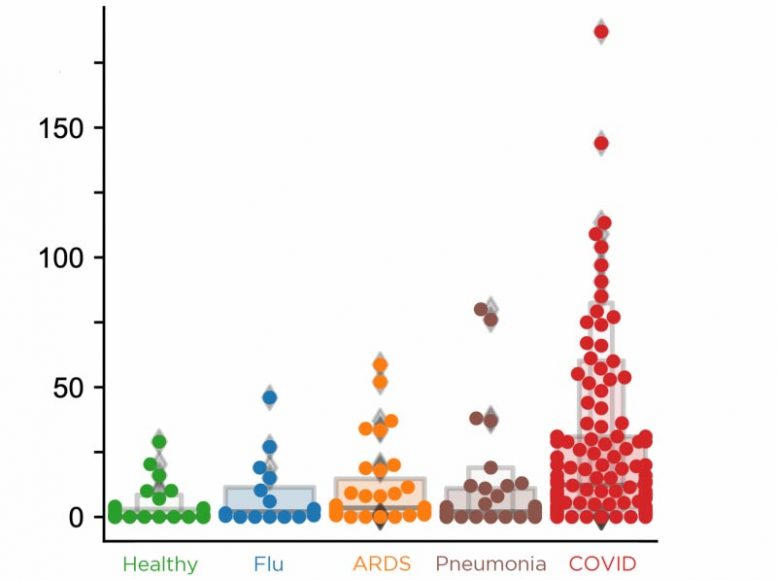
The lungs of clients with COVID-19 have more monocytes revealing IL-1beta than lungs from clients with other breathing conditions. Credit: Benjamin Izar.
“In COVID-19, we see expansion and uncontrolled activation of macrophages, including alveolar macrophages and monocyte-derived macrophages,” Izar states. “They are completely out of balance and allow inflammation to ramp up unchecked. This results in a vicious cycle where more immune cells come in causing even more inflammation, which ultimately damages the lung tissue.”
One inflammatory cytokine in specific, IL-1ß, is produced at a high rate by these macrophages.
“Unlike other cytokines such as IL-6, which appears to be universally prevalent in various pneumonias, IL-1ß production in macrophages is more pronounced in COVID-19 compared to other viral or bacterial lung infections,” Izar states. “That’s important because drugs exist that tamp down the effects of IL-1ß.”
Some of these drugs are currently being checked in scientific trials of COVID clients.
Severe COVID likewise avoids lung repair work
In a common infection, an infection damages lung cells, the body immune system clears the pathogen and the particles, and the lung restores.
But in COVID, the brand-new research study discovered that not just does SARS-CoV-2 infection ruin alveolar epithelial cells crucial for gas exchange, the occurring swelling likewise hinders the capability of the staying cells to restore the harmed lung.
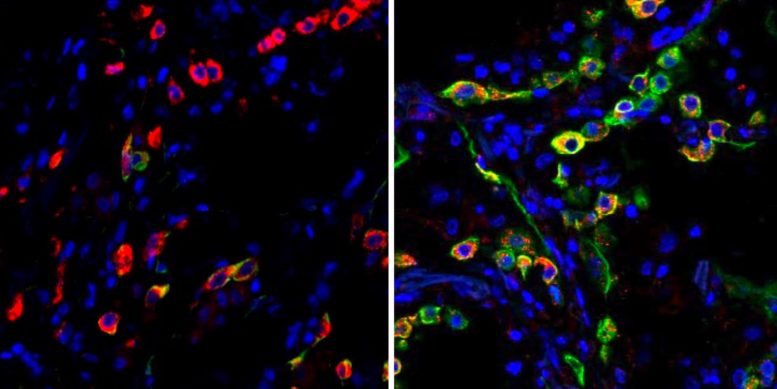
Lung cells in clients with serious COVID end up being caught in a state (suggested by the green color) that avoids the cells from fixing damage triggered by the infection. The left image programs cells from a healthy lung; the ideal image reveals lung cells from a client who passed away from COVID-19. Credit: Benjamin Izar / Columbia University Vagelos College of Physicians and Surgeons.
Though the lung still consists of cells that can do the repair work, swelling completely traps these cells in an intermediate cell state and leaves them not able to finish the last actions of distinction required for replacement of fully grown lung epithelium.
“Among others, IL-1ß appears to be a culprit in inducing and maintaining this intermediate cell state,” states Izar, “thereby linking inflammation and impaired lung regeneration in COVID-19. This suggests that in addition to reducing inflammation, targeting IL-1ß may help take the brakes off cells required for lung repair.”
Preventing sped up fibrosis
The scientists likewise discovered a a great deal of particular fibroblast cells, called pathological fibroblasts, that produce quick scarring in COVID-19 lungs. When the fibroblast cells fill the lung with scar tissue, a procedure called fibrosis, the lung has less area for cells associated with gas exchange and is completely harmed.
Given the significance of pathological fibroblasts in the illness, Izar’s group carefully examined the cells to reveal prospective drug targets. An algorithm called VIPER, established formerly by Andrea Califano, Dr, chair of systems biology at Columbia University Vagelos College of Physicians and Surgeons, determined numerous particles in the cells that play an essential function and might be targeted by existing drugs.
“This analysis predicted that inhibition of STAT signaling could alleviate some of the deleterious effects caused by pathological fibroblasts,” Izar states.
“Our hope is that by sharing this analysis and massive data resource, other researchers and drug companies can begin to test and expand on these ideas and find treatments to not only treat critically ill patients, but also reduce complications in people who survive severe COVID-19.”
Team effort by numerous Columbia laboratories
“Pulling this study together in such a short period of time was only possible with the help of several teams of researchers at Columbia,” Izar states.
Critically, in the very first couple of months of the pandemic, Columbia’s Department of Pathology & Cell Biology chose to flash-freeze numerous tissues from departed COVID clients to protect the cells’ molecular state. Hanina Hibshoosh, MD, director of the department’s tissue bank, started the cooperation with Izar’s laboratory, which has know-how in performing single-cell analyses with frozen tissue. Pathologist Anjali Saqi, MD, teacher of pathology & cell biology, was likewise crucial in acquiring and assessing the samples.
Jianwen Que, MD, PhD, teacher of medication, and his lab supplied know-how in recognizing and defining cells in the lung and their regenerative capacity. Fibrosis specialist Robert Schwabe, MD, associate teacher of medication, was necessary in dissecting systems by which COVID-19 moved lung scarring.
“We are incredibly grateful to all the labs contributing to this effort and very fortunate to be at Columbia with all the necessary expertise at hand in one collaborative environment,” Izar states.
Reference: “A molecular single-cell lung atlas of lethal COVID-19” by Johannes C. Melms, Jana Biermann, Huachao Huang, Yiping Wang, Ajay Nair, Somnath Tagore, Igor Katsyv, André F. Rendeiro, Amit Dipak Amin, Denis Schapiro, Chris J. Frangieh, Adrienne M. Luoma, Aveline Filliol, Yinshan Fang, Hiranmayi Ravichandran, Mariano G. Clausi, George A. Alba, Meri Rogava, Sean W. Chen, Patricia Ho, Daniel T. Montoro, Adam E. Kornberg, Arnold S. Han, Mathieu F. Bakhoum, Niroshana Anandasabapathy, Mayte Suárez-Fariñas, Samuel F. Bakhoum, Yaron Bram, Alain Borczuk, Xinzheng V. Guo, Jay H. Lefkowitch, Charles Marboe, Stephen M. Lagana, Armando Del Portillo, Emmanuel Zorn, Glen S. Markowitz, Robert F. Schwabe, Robert E. Schwartz, Olivier Elemento, Anjali Saqi, Hanina Hibshoosh, Jianwen Que and Benjamin Izar, 29 April 2021, Nature.
DOI: 10.1038/s41586-021-03569-1
The scientists were supported by the U.S. National Institutes of Health (grants K08CA222663, U54CA225088, R37CA258829, R01HL152293, R01HL132996, T32CA203702, UL1TR002384, R01CA194547, R01CA234614, R01AI107301, R01DK121072 and R03DK117252); QuickGrants; a Burroughs Wellcome Fund Career Award for Medical Scientists; the Louis V. Gerstner Jr. Scholars Program; the U.S. Department of Defense (Discovery Award PR200616); Volastra, Janssen, and Eli Lilly research study grants; the Leukemia and Lymphoma Society (grants SCOR 7012-16, SCOR 7021-20, and SCOR 180078-02); an Irma Hirschl Trust Research Award Scholar; and the Damon Runyon Cancer Research Foundation (DRQ-03-20).
This research study was moneyed in part through the NIH Support Grant S10RR027050 for circulation cytometry analysis and the NIH/NCI Cancer Center Support Grant P30CA013696 at Columbia University’s Genetically Modified Mouse Model Shared Resource, Molecular Pathology Shared Resource, and Tissue Bank.
Benjamin Izar is an expert for Merck and Volastra Therapeutics. Olivier Elemento is clinical consultant and equity holder in Freenome, Owkin, Volastra Therapeutics, and One3 Biotech. Robert E. Schwartz belongs to the clinical board of advisers of Miromatrix Inc. Daniel T. Montoro is an expert for LASE Innovation.