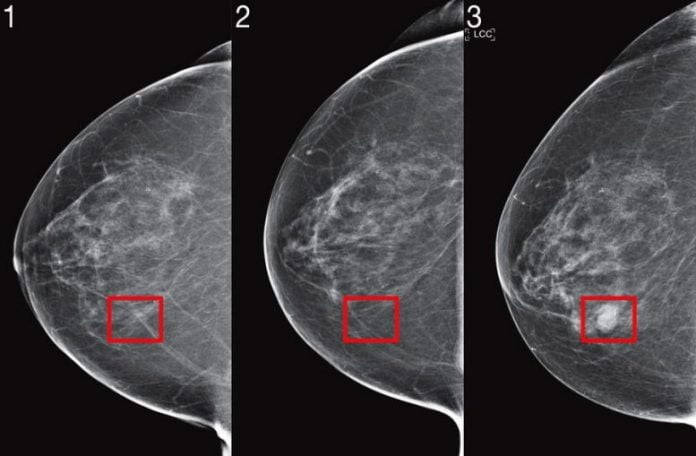
MIT scientists have actually enhanced their device finding out system established to anticipate cancer threat from mammogram images, and verified their efficiency with research studies throughout numerous medical facilities. Credit: Images thanks to the scientists
Researchers produced a risk-assessment algorithm that reveals constant efficiency throughout datasets from United States, Europe, and Asia.
To catch cancer previously, we require to anticipate who is going to get it in the future. The intricate nature of forecasting threat has actually been boosted by expert system (AI) tools, however the adoption of AI in medication has actually been restricted by bad efficiency on brand-new client populations and disregard to racial minorities.
Two years earlier, a group of researchers from MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) and Jameel Clinic (J-Clinic) showed a deep knowing system to anticipate cancer threat utilizing simply a client’s mammogram. The design revealed considerable pledge and even enhanced inclusivity: It was similarly precise for both white and Black females, which is specifically crucial considered that Black females are 43 percent most likely to pass away from breast cancer.
But to incorporate image-based threat designs into scientific care and make them commonly readily available, the scientists state the designs required both algorithmic enhancements and massive recognition throughout numerous medical facilities to show their toughness.
To that end, they customized their brand-new “Mirai” algorithm to catch the special requirements of threat modeling. Mirai collectively designs a client’s threat throughout several future time points, and can additionally take advantage of scientific threat elements such as age or household history, if they are readily available. The algorithm is likewise developed to produce forecasts that correspond throughout small variations in scientific environments, like the option of mammography device.
Robust expert system tools might be utilized to anticipate future breast cancer.
The group trained Mirai on the exact same dataset of over 200,000 examinations from Massachusetts General Hospital (MGH) from their previous work, and verified it on test sets from MGH, the Karolinska Institute in Sweden, and Chang Gung Memorial Hospital in Taiwan. Mirai is now set up at MGH, and the group’s partners are actively dealing with incorporating the design into care.
Mirai was substantially more precise than previous approaches in anticipating cancer threat and recognizing high-risk groups throughout all 3 datasets. When comparing high-risk associates on the MGH test set, the group discovered that their design recognized almost 2 times more future cancer medical diagnoses compared the present scientific requirement, the Tyrer-Cuzick design. Mirai was likewise precise throughout clients of various races, age, and breast density classifications in the MGH test set, and throughout various cancer subtypes in the Karolinska test set.
“Improved breast cancer risk models enable targeted screening strategies that achieve earlier detection, and less screening harm than existing guidelines,” states Adam Yala, CSAIL PhD trainee and lead author on a paper about Mirai that was released recently in Science Translational Medicine. “Our goal is to make these advances part of the standard of care. We are partnering with clinicians from Novant Health in North Carolina, Emory in Georgia, Maccabi in Israel, TecSalud in Mexico, Apollo in India, and Barretos in Brazil to further validate the model on diverse populations and study how to best clinically implement it.”
How it works
Despite the large adoption of breast cancer screening, the scientists state the practice is filled with debate: More-aggressive screening methods intend to optimize the advantages of early detection, whereas less-frequent screenings intend to lower incorrect positives, stress and anxiety, and expenses for those who will never ever even establish breast cancer.
Current scientific standards utilize threat designs to identify which clients ought to be advised for extra imaging and MRI. Some standards utilize threat designs with simply age to identify if, and how frequently, a female ought to get evaluated; others integrate several elements connected to age, hormonal agents, genes, and breast density to identify additional screening. Despite years of effort, the precision of threat designs utilized in scientific practice stays modest.
Recently, deep knowing mammography-based threat designs have actually revealed appealing efficiency. To bring this innovation to the center, the group recognized 3 developments they think are vital for threat modeling: collectively modeling time, the optional usage of non-image threat elements, and approaches to guarantee constant efficiency throughout scientific settings.
1. Time
Inherent to run the risk of modeling is gaining from clients with various quantities of follow-up, and examining threat at various time points: this can identify how frequently they get evaluated, whether they ought to have extra imaging, or perhaps think about preventive treatments.
Although it’s possible to train different designs to evaluate threat for each time point, this technique can lead to threat evaluations that don’t make good sense — like anticipating that a client has a greater threat of establishing cancer within 2 years than they do within 5 years. To address this, the group developed their design to anticipate threat at all time points all at once, by utilizing a tool called an “additive-hazard layer.”
The additive-hazard layer works as follows: Their network anticipates a client’s threat at a time point, such as 5 years, as an extension of their threat at the previous time point, such as 4 years. In doing so, their design can gain from information with variable quantities of follow-up, and after that produce self-consistent threat evaluations.
2. Non-image threat elements
While this approach mainly concentrates on mammograms, the group wished to likewise utilize non-image threat elements such as age and hormone elements if they were readily available — however not need them at the time of the test. One technique would be to include these elements as an input to the design with the image, however this style would avoid most of medical facilities (such as Karolinska and CGMH), which don’t have this facilities, from utilizing the design.
For Mirai to take advantage of threat elements without needing them, the network anticipates that details at training time, and if it’s not there, it can utilize its own predictive variation. Mammograms are abundant sources of health details, therefore numerous standard threat elements such as age and menopausal status can be quickly anticipated from their imaging. As an outcome of this style, the exact same design might be utilized by any center internationally, and if they have that extra details, they can utilize it.
3. Consistent efficiency throughout scientific environments
To integrate deep-learning threat designs into scientific standards, the designs should carry out regularly throughout varied scientific environments, and its forecasts cannot be impacted by small variations like which device the mammogram was handled. Even throughout a single medical facility, the researchers discovered that basic training did not produce constant forecasts prior to and after a modification in mammography devices, as the algorithm might discover to count on various hints particular to the environment. To de-bias the design, the group utilized an adversarial plan where the design particularly discovers mammogram representations that are invariant to the source scientific environment, to produce constant forecasts.
To additional test these updates throughout varied scientific settings, the researchers assessed Mirai on brand-new test sets from Karolinska in Sweden and Chang Gung Memorial Hospital in Taiwan, and discovered it acquired constant efficiency. The group likewise examined the design’s efficiency throughout races, ages, and breast density classifications in the MGH test set, and throughout cancer subtypes on the Karolinska dataset, and discovered it carried out likewise throughout all subgroups.
“African-American women continue to present with breast cancer at younger ages, and often at later stages,” states Salewai Oseni, a breast cosmetic surgeon at Massachusetts General Hospital who was not included with the work. “This, coupled with the higher instance of triple-negative breast cancer in this group, has resulted in increased breast cancer mortality. This study demonstrates the development of a risk model whose prediction has notable accuracy across race. The opportunity for its use clinically is high.”
Here’s how Mirai works:
1. The mammogram image is executed something called an “image encoder.”
2. Each image representation, in addition to which see it originated from, is aggregated with other images from other views to acquire a representation of the whole mammogram.
3. With the mammogram, a client’s standard threat elements are anticipated utilizing a Tyrer-Cuzick design (age, weight, hormone elements). If not available, anticipated worths are utilized.
4. With this details, the additive-hazard layer anticipates a client’s threat for each year over the next 5 years.
Improving Mirai
Although the present design doesn’t take a look at any of the client’s previous imaging outcomes, modifications in imaging with time include a wealth of details. In the future the group intends to produce approaches that can successfully make use of a client’s complete imaging history.
In a comparable style, the group keeps in mind that the design might be even more enhanced by using “tomosynthesis,” an X-ray strategy for evaluating asymptomatic cancer clients. Beyond enhancing precision, extra research study is needed to identify how to adjust image-based threat designs to various mammography gadgets with minimal information.
“We know MRI can catch cancers earlier than mammography, and that earlier detection improves patient outcomes,” states Yala. “But for patients at low risk of cancer, the risk of false-positives can outweigh the benefits. With improved risk models, we can design more nuanced risk-screening guidelines that offer more sensitive screening, like MRI, to patients who will develop cancer, to get better outcomes while reducing unnecessary screening and over-treatment for the rest.”
“We’re both excited and humbled to ask the question if this AI system will work for African-American populations,” states Judy Gichoya, MD, MS and assistant teacher of interventional radiology and informatics at Emory University, who was not included with the work. “We’re extensively studying this question, and how to detect failure.”
Reference: “Toward robust mammography-based models for breast cancer risk” by Adam Yala, Peter G. Mikhael, Fredrik Strand, Gigin Lin, Kevin Smith, Yung-Liang Wan, Leslie Lamb, Kevin Hughes, Constance Lehman and Regina Barzilay, 27 January 2021, Science Translational Medicine.
DOI: 10.1126/scitranslmed.aba4373
Yala composed the paper on Mirai along with MIT research study expert Peter G. Mikhael, radiologist Fredrik Strand of Karolinska University Hospital, Gigin Lin of Chang Gung Memorial Hospital, Associate Professor Kevin Smith of KTH Royal Institute of Technology, Professor Yung-Liang Wan of Chang Gung University, Leslie Lamb of MGH, Kevin Hughes of MGH, senior author and Harvard Medical School Professor Constance Lehman of MGH, and senior author and MIT Professor Regina Barzilay.
The work was supported by grants from Susan G Komen, Breast Cancer Research Foundation, Quanta Computing, and the MIT Jameel Clinic. It was likewise supported by Chang Gung Medical Foundation Grant, and by Stockholm Läns Landsting HMT Grant.